|
Scientist
>
Prof. Dr. Gerd Liebezeit
>
Environmental microplastics
Environmental microplasticsA danger to human health?Humans create their own environment and since the 1950s, this has increasingly included products made from synthetic polymers, commonly referred to as "plastics". Plastic waste in the environment is probably here to stay for decades – if not centuries. But can we actually remove plastics – and microplastics in particular – from the environment?
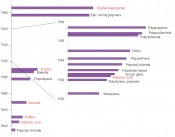
While such compounds were known of before the establishment of polymer science (Fig.1), the decisive structural propositions were put forward by Hermann Staudinger in 1920 [1]. From a paltry 1.7million tonnes in 1950, global plastic production has risen by an average of 0.9% annually to reach 299 million tonnes in 2013, with 57million tonnes being produced in Europe (EU except Croatia, plus Switzerland and Norway; source: www.plasti A considerable percentage of global polymer production is destined for use in disposable products, primarily packaging (about 40% in the EU). While the European average for plastic waste in landfill sites is only 38%, there is a marked North-South and East-West gradient. Accordingly, some 37% to 87% of this waste ends up in official landfill sites in countries that still allow this disposal route to be used for plastics. While figures on illegal tipping are naturally not available, appearances suggest that this disposal route can make a major contribution to the eyesore of countryside littering in several European countries. Of the 275million tonnes of plastic waste generated by the 192 coastal states, figures from Jambeck et al. [2] suggest that annually between 4.8 and 12.7 million tonnes end up in the ocean (data for 2010). The greatest advantage of products made from synthetic polymers is at the same time their most problematic feature in environmental terms – namely their durability. And the resistance of plastics to microbial degradation is indeed one of their most critical attributes in both an aquatic and terrestrial context. It can take up to 600 years for some types of plastics to disappear from the environment (Table 1).
Tab. 1: Estimated degradation times for various polymers in an aquatic environment, from the literature
The actual rate of degradation will depend on the formulation of the polymer and actual environmental conditions, however. In interpreting these values, we must also bear in mind that they have generally been obtained by determining the loss of mechanical properties as a result of ageing: end-stage degradation to water and carbon dioxide has been infrequently studied to date.
Fig. 1: Developments in polymers – red: some synthetics based on biological polymers
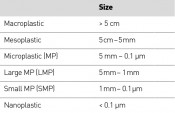
Where do microplastics come from? The first stage in the process always involves the fragmentation of environmental macroplastics into smaller-scale particles – i.e. to mesoplastics, microplastics and nanoplastics (Table 2, Fig. 2). According to figures from the Federal Environment Agency, 65 plastic bags are used per person per year in Germany. This equates to an annual consumption of 5.3 billion plastic bags – or 10,000 bags a minute. Alongside Spain and the UK, Germany leads the rest of the EU in terms of plastic bag consumption.
Tab. 2:Size classes for various categories of plastic waste:
Fig. 2: From macro- to microplastics
These figures not only include the illegal littering of plastic bags, beverage bottles or fast food packaging: mulch film from the agricultural and horticultural sectors is also a contributor to particle pollution. Mulching films are typically made from polyethylene, which is naturally resistant to degradation. Accordingly, metal salts (such as cobalt compounds) are added to ensure the product breaks down when exposed to ultraviolet radiation and oxygen. Recycled polystyrene (expanded polystyrene) and urea formaldehyde resin waste is used as a soil additive with particle sizes of only a few millimetres. While the solid PS improves soil air circulation, the porous resin increases the capacity for water retention. Polystyrene is (intentionally) very light (density 15 to 90 mg/cm³), however, and can be easily scattered into the environment by the wind (Fig. 3).
Fig. 3:Polystyrene waste "scum" from a 7cm flower pot
Biological processes can also lead to microplastic pollution. Marine isopods can gnaw holes into polystyrene floats to create burrows or havens from predators; marine birds also increase microplastic pollution by pecking at macroplastic waste [3]. The focus of such activities will often be a piece of expanded polystyrene (Fig.4).
Fig. 4: Expanded polystyrene float ball (left) and block (right) with peck marks made by large gulls – Kachelotplate sandbar, East Frisian Islands, and Mellum, German Bight – 2012
While these degradation processes occur within the environment, microplastics can also enter the environment directly. Sources here include cosmetics and detergents containing particles (typically made from polyethylene but polypropylene or polyamide polishing agents are also found) and metalworking abrasives, used as a substitute for the traditional sand in surface finishing treatments. Every piece of clothing sheds fibres during wearing, washing and drying. Wearing is clearly responsible for the largest proportion of this shedding, even if exact figures are not available. When an article of polyester or polyacrylic clothing is washed, it sheds around 0.01–0.06% (by weight) of its fibres. Carried in waste water to treatment plants, these fibres are not fully filtered out and so end up being released with the treated water into the environment [4]. If the sewage sludge – which initial measurements tell us contains the bulk of the fibres – is then used as an agricultural fertiliser, the retained fibres can be released into the atmosphere and be distributed very widely indeed. Airborne fibres can be deposited on any surface, including flower petals – and thus end up in honey, for example [5].
Thermoplastic synthetics are shipped as raw materials in a form known as “pre-production pellets”. A few millimetres in size, these granular particles can be lost during loading and thus end up in the environment (www.pellet Microplastics can thus be found everywhere: in the air, in the water, in soils and in aquatic sediments. From here, they can enter food and potable water and be taken up by organisms. What risks are posed by microplastics?
While synthetic polymers are generally harmless in their pure form, two other factors turn them into an environmental hazard. First, plastics can contain traces of monomers such as bisphenol A (used as starting material for polycarbonates, for example) and di-/tri-styrene or styrene. Second, almost all synthetic polymers will contain one or more additives. Obvious candidates here include brominated flame retardants and plasticisers – especially phthalates, which in PVC products can make up as much as 60% of the product's weight. Many of these additives have hormonal effects. In aqueous media, they leach out of plastics and thus end up in the environment (see www.lfu.ba Generally, plastic is hydrophobic. This allows e.g. polyethylene films to be used as long-term collectors of lipophilic organic contaminants such as PCBs, PAHs or DDT, DDE and DDD. Since microplastics also have a high surface-to-volume ratio, they can also adsorb very large quantities of such pollutants [5]. If these kinds of contaminated particles are ingested by organisms, the pollutants (and additives) can be released into the stomach or digestive tract, become stored in the animal's tissue and trigger health problems [6]. And, as various lab studies have shown, even uncontaminated microplastics are problematic: when small enough – roughly < 5m – they can be taken up by zooplankton, common mussels or lugworms and become incorporated in tissue, where they can cause inflammatory reactions [7]. Even larger-sized, microplastics can still be ingested by organisms and passed up the food chain. In the first stage, microplastics are eaten by zooplankton. From here, they are passed on to mussels, fish, marine birds or marine mammals such as harbour seals, grey seals or porpoises. Microplastics have also been discovered in sediment-dwelling or -ingesting animals such as lugworms and sea cucumbers [8]. While data for humans are not yet available, the negative effects of tiny particles taken up via the airways (for example) have been known for a long time (asbestosis, silicosis). Many marine birds look for food on the surface of the water and will try to consume anything they find there, including plastic. As omnivores, seagulls not only eat fish, crustaceans or mussels but will also hunt for food on land – often visiting rubbish dumps, for example. This type of scavenging leads to the consumption of both macro- and microplastics, which they regurgitate [9] and which become especially concentrated in bird colonies. Fulmars grind up plastic they consume with their gizzards, thereby contributing to the production of microplastics. Outlook Plastic waste in the environment is probably here to stay for decades – if not centuries. But can we actually remove plastics – and microplastics in particular – from the environment? Unlike the macroplastic problem, the collection of tiny pieces of plastic is an untenable alternative, and filtering microplastics out of rivers, seas or oceans with small-mesh nets would mean wholesale interference in aquatic ecosystems, since the technique would also remove zooplankton and thus the base of the food chain. Pressure from environmental groups and consumers has led to the banning of microplastic polishing agents from toothpastes in Germany, and almost all large cosmetic producers have stated – after huge pressure from environmental groups and consumers – that they will soon be phasing out microplastics in exfoliants and similar products. The option of banning plastic bags now being discussed by many local authorities is also certainly helping to raise public awareness about the problem of plastic waste of all sizes. Operators of water treatment plants must also be required by law to install facilities capable of removing microscopic fibres from waste water. Despite all of the above, the long lifetimes estimated for environmental plastic will also require political decision-making to reduce the tidal wave of plastics, e.g. by bans on disposable packaging (and plastic bags in particular) – as currently being discussed within the EU. As consumers, we can all take action to reduce plastic waste, however. There are plenty of alternatives for cosmetics, clothing, packaging and other consumer goods.
Bibliography Picture: © istockphoto.com| Pgiam, Andrey Danilovich |
L&M int. 3 / 2015
Free download here: download here The Author:Read more articles online |