|
Tropical pitcher plant and biomimetics
Tropical pitcher plant and biomimeticsHierarchical anti-adhesive surfaces by mimiking insect trapsCarnivorous plants, among them tropical pitcher plants (Nepenthes spp.), have always fascinated people due to their remarkable ability to feed on animals and have constantly drawn the attention of researches as soon as new species were discovered. During past decades, different aspects of Nepenthes biology, among them the structure and functions of trapping organs (pitchers), particularly with respect to their trapping efficiency, have been the focus of numerous field observations and structural and experimental studies. Very recently, these plants are also used as model systems for various biomimetic applications. The current cooperative project between the Zoological Institute at the Kiel University and the Institute of Chemistry of New Materials at the University of Osnabrueck aims at the development of hierarchical anti-adhesive materials by mimicking the slippery zone in N. alata pitchers. Catch and trap Carnivorous (animal eating) plants represent an unusual group of organisms, which grow in habitats with very poor soils and rely on nutrients derived from captured animals, mostly insects. In their evolution, these plants have turned some of their leaves into specialised organs, which fulfil the trapping function. These trapping organs, being extremely diverse, all serve for attracting, trapping and retaining animals, digesting them and absorbing prey-derived nutrients. Some traps use different movements to capture animals, as for example in the venus fly trap (Dioneae muscipula) acting very fast or in sundews (Drosera spp.) with slowly moving tentacles. Another group of carnivorous plants have passive trapping organs, which employ no movement for capturing animals and work like a simple pitfall, lobster pot or fly paper. Pitcher-shaped trapping organs, which are produced at the tips of tendrils and use a passive pitfall mechanism for capturing, are characteristic for carnivorous plants from the genus Nepenthes (Fig.1). These pitchers consist of several structural and functional zones having specialised macroand micromorphological features and serving different functions. The slippery zone, situated in the upper part inside the pitcher (Fig. 2), was recognised long ago as an important structure for insect trapping and retention, due to its particular moon-shaped cells called lunate cells and an epicuticular three-dimensional wax coverage.
Fig. 1: Pitchers from several Nepenthes species. A. N. alata. B. N. fusca. C. N. macrophylla. D. N. mirabilis. E. N. rafflesiana. F. N. dicksoniana. G. N. ventricosa. H. N. veitchii. I. N. bicalcarta.The dashed lines show the length of the slippery zone.
Fig. 2: Longitudinal section through a pitcher of Nepenthes.
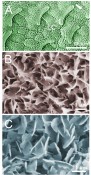
Slipperiness perfected Intensive micromorphological studies performed in our group during last several years revealed that in the model species N. alata, this zone contains three distinct levels of hierarchically arranged surface structures: (1) anisotropic lunate cells (length scale of several dozens micrometers); (2) the lower wax layer composed of interconnected microscopic wax crystals and (3) the upper wax layer consisting of separate microscopic plate-like wax crystals (Fig. 3).
Fig. 3: Hierarchically aligned surface structures in the slippery zone of Nepenthes alata pitchers (scanning electron microscopy). A. Pitcher surface with lunate cells (LC) and wax coverage. The arrow indicates the location of the pitcher floor. B. Lower wax layer. C. Upper wax layer.
Fig. 4: Superhydrophobic properties of the slippery zone in Nepenthes alata pitchers. A. Contact angle of water. B. Surface after wetting with water (cryo-scanning electron microscopy). WD = water drops.
These structural hierarchical levels play different roles in the reduction of insect attachment to the slippery zone due to different physical mechanisms. Although the potential role of downward- directed lunate cells in hindering insect attachment by preventing claw interlocking was first suggested nearly a century ago by Knoll, his experiments could not show an anisotropic effect on insect locomotion. We have recently performed the experimental study estimating attachment forces of ladybird beetles Coccinella septempunctata on pitchers of the model plant species N. alata and demonstrated for the first time that the particular shape and distribution of lunate cells cause anisotropic frictional properties of the surface. Tests on pitcher samples and their polymer replicas revealed that just claws were responsible for attachment enhancement in the downward pitcher direction, where inverted lunate cells served as anchorage sites for claws. Moving in an upward direction, as in a natural situation, when insects try to escape from the trap, they are unable to employ their claws, because of the downward orientation of overhanging edges of lunate cells. Two superimposed wax layers presenting the second and third hierarchical levels of the slippery zone differ not only in their structure, but also in chemical composition and mechanical properties. The lower layer resembles foam, composed of interconnected membraneous platelets protruding from the surface at some acute angles, and is rather stable mechanically. The upper layer consists of separate irregular platelets that are located not like roof tiles as suggested previously, but more or less perpendicular to the subjacent layer. Upper crystals are connected to the lower layer through long slim stalks, which can break very easily. Although waxes from both layers are composed of the same compound classes, the latter occur in different proportions. Chain length distributions in aliphatic compounds also differ in extracts from the lower and the upper layers. Both wax layers demonstrate superhydrophobic properties (Fig. 4) and are almost equally unwettable by polar liquids (water and ethylene glycol). However, contact angles of non-polar fluid diiodomethane (both >100) show a significant difference between layers. When unstructured, these waxes exhibit similar material properties (elasticity modulus and hardness), but in native structured state, the lower layer is harder and stiffer than the upper one. Moreover, crystals of the upper layer are rather brittle and may be easily exfoliated or broken into tiny pieces. Ladybird traction tests Both wax layers prevent insect adhesion. We carried out force tests with two species of ladybird beetles, Adalia bipunctata and Coccinella septempunctata, and found a dramatic force reduction on the waxy surface compared to reference substrates such as glass and wax-free pitcher surfaces or their polymer replicas. Interestingly, the comparison of force values between two wax layers showed no significant difference: thus, both hierarchical levels based on wax crystals have similar impact on insect attachment. On these surfaces due to the fragile and brittle nature of wax crystals and their small dimensions, insects cannot apply their claws for interlocking with crystals in order to climb up the pitcher wall. The adhesion by means of adhesive pads is minimized using different mechanisms: via reduction of the real contact area caused by surface microroughness (lower layer) and contamination of insect adhesive pads by wax crystals (upper layer). This was found out after examination of beetles’ pads just after force tests: the upper layer caused pad contamination with wax material (Fig. 5), whereas the lower layer did not.
Fig. 5: Contamination of insects' adhesive organs by wax crystals from the upper layer of the slippery zone from Nepenthes alata pitchers (scanning electron microscopy). A. Functional adhesive structures of Adalia bipunctata beetles B. Adhesive structures after contact with the slippery zone.
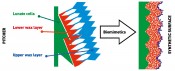
Adhesive properties of two wax layers are very recently studied by applying a novel method for adhesion measurements on anti-adhesive surfaces. For this purpose, we used half-spheres made of tacky polydimethylsiloxane as probes and measured pull-off forces. Keeping applied force in the range corresponding to pressure caused by small insects, we obtained the lowest adhesion force on the upper wax layer compared to both the lower wax layer and reference microrough polymer surface having similar roughness parameters as the slippery zone. Like in our previous experiments with beetles, we observed strong contamination of adhesive halfspheres by wax crystals, detached from the upper layer and adhering to the halfspheres during tests, whereas no contaminating effects were detected for other test surfaces. New anti-adhesive synthetic surfaces Inspired by the complex structure of the N. alata pitcher, we are designing bioinspired anti-adhesive surfaces for technical applications (Fig.6).
Fig. 6: A biomimetic approach: Development of hierarchically organised anti-adhesive materials that imitate the complex structure of the slippery zone in Nepenthes alata pitchers.
Such surfaces might pave the way for novel types of artificial surfaces that repel dust, sticky dirt, moisture and insects. Strategies to produce bioinspired anti-adhesive surfaces should allow up-scaling so that high-throughput production of large areas is possible. To meet these requirements, the first biological level (lunate cells) is mimicked by selfassembled monolayers of microspheres with sizes ranging from a few micrometers to several tens of micrometers (Fig.7), assuming that the anisotropy of the biological model is not required for most technical applications.
Fig. 7: Biomimetic structure of the first hierarchical layer, developed by replication of polystyrene microspheres with a diameter of 25m. A. Light microscopy with backlight. B. Scanning electron microscopy. C. 2D image using white light interferometer D. 3D image using white light interferometer.
The challenge is rather to minimize the contact area between the anti- adhesive surface and any kind of counterpart surface. To this end, we have at first developed a method for the high-throughput replication of the microsphere monolayers. A large number of master moulds is accessible by a two-step replication process from one microsphere monolayer, serving as sacrificial primary template. Each master mould can in turn be replicated many times. The next step is to minimize adhesion of the anti-adhesive surfaces to rigid counterpart surfaces forming contact only with tips and protrusions as well as to soft, elastic counterpart surfaces. At the moment, several types of surface topographies are tested with respect to their antiadhesive properties to rigid and elastic counterpart surfaces. Moreover, the implementation of biological levels 2 and 3 is on the way. Level 2 will be introduced by embossing the microspheres with nanoporous alumina moulds accessible by special anodization procedures or by replication of the microspheres with nanoporous materials, having bicontinuous morphology. These nanoporous materials can be obtained via phase separation processes. Level 3 will be implemented by dispersing waxy nanorods, which can be made using the above mentioned nanoporous alumina moulds, on combined artificial level1/level2 structures. This work was partly supported by the German Science Foundation DFG (Priority Pro-gramme 1420 “Biomimetic Materials Research: Functionality by Hierarchical Structuring of Materials”, research grants GO 995/9-1, GO 995/9-2, STE 1127/12-1 and STE 127/12-2).
Bibliography Picture: © panthermedia | cbenjasuwan |
L&M int. 3 / 2014
Free download here: download here The Authors:Read more articles online |