|
L&Mint-int-4-2014
>
Precisely targeted regulation
Precisely targeted regulationlab&more in conversation with Stefan Miltenyi, CEO of Miltenyi Biotec GmbH, Germany
The biomedical community is euphoric about recent results in clinical trials of new treatment modalities for genetic diseases and oncological indications: T cells with modified chimeric antigen receptors (CAR) have been genetically modified with the aid of lentiviral technology and hold out the prospect of a novel form of cancer immunotherapy. Lentiviral technology itself is currently regarded as the most effective way of inserting genetic material into a cell in order to modulate its function. Miltenyi Biotec GmbH, which is one of the oldest and largest biotechnology companies in Germany, announced in August that they had acquired the lentivirus business segment of the US company, Lentigen Corporation.
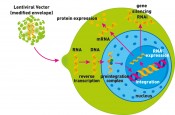
Transduction mechanism of lentiviral vectors. The lentiviral vector transfers genetic material into the target cell, where it is inserted into the host genome. This can lead to the expression of a particular protein. Alternatively, the expression of a particular protein can be inhibited through formation of a specific RNA molecule (RNAi).
Mr. Miltenyi, what are the advantages that lentiviral vectors have over other gene therapy vectors that made you decide to acquire the lentiviral production technology of one of the leaders in this field? The main objectives of all gene therapy vectors are to deliver a specific genetic payload safe and efficiently to target cells where the transgene is to be expressed. In contrast to other gene delivery systems lentiviral vectors can transduce target cells very efficiently with very little toxicity. A gene of interest is then stably expressed in the transduced cell and in its daughter cells. This is required if the transgene has to be expressed for a prolonged time (e.g. for cancer therapy). Moreover, lentiviral vectors serve as a very broad gene delivery platform as pseudotyped vectors can transduce all types of mammalian cells, including non-dividing cells which cannot be transduced with gamma-retroviral vectors. Whenever viruses are discussed, their ability to mutate is usually a central topic, especially in the case of retroviruses. What precautions are being taken in your company to prevent replication-competent viruses from being formed? In contrast to the genome of a virus that contains all viral genes, the genome of a lentiviral vector is modified so that every viral gene is deleted or truncated, which prevents the formation of new viral particles upon transduction. For the production of lentiviral vectors the viral components are split on several different constructs, which then are added in trans – a system called split packaging. During all the years of experience with lentiviral vectors, a replication-competent lentivirus has never been detected. Lentiviral vectors are generated from the master copy of the plasmids used for split packaging, and subsequent cell transduction is a single event. Therefore, there is no accumulation of mutations as in the case of a replicating virus. Probably the two greatest challenges for gene therapy are to guide the integration of the therapeutic gene into the host DNA correctly and to prevent the expression of other genes on the host chromosome from being changed by the integration process. What progress has been made to meet these challenges and how effective is your vector system? One of the features of lentiviral vectors is their ability to efficiently deliver their payloads into the genome of target cells. Unlike gamma-retroviral vectors, which contain strong promoters that can activate neighboring enhancer elements, such as oncogenes, lentiviral vectors do not contain strong promoters to upregulate their expression. The lack of strong promoters in lentiviral vectors prevents the genotoxic effects seen with the gamma-retroviral vectors. In addition, the 3’ LTR has been deleted to generate vectors with further decreased genotoxic potential. Several pre-clinical studies have confirmed that lentiviral vectors have no genotoxic potential, in contrast to gamma-retroviral vectors. Also the accumulating safety data from various clinical trials further support these findings. In the future, we anticipate to have a range of lentiviral vector products that address the evolving needs of the translational medicine scientist. These include various pseudotypes and commercial-scale manufacturing. What are the new therapeutic options that will be made available through lentiviral technology?
The technology will provide a robust method for stable gene delivery into mammalian cells of interest. These include hematopoietic cells, such as T?cells provide, as an example, new options for cancer therapy, as indicated by the promising results seen with CAR-T cell therapy for acute lymphocytic leukemia. HSCs provide therapeutic options for a variety of diseases, including genetic diseases like ALD, beta thalassemia and SCID. This is just the beginning for therapeutic applications of this technology. (Interview: Claudia Schiller) |
L&M int. 4 / 2014
Free download here: download here |