|
L&Mint-int-4-2014
>
Cancer prevention and nutrition
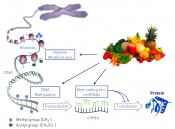
Cancer prevention and nutritionWhat role does epigenetics play?Detoxification, anti-inflammatory agents, radical scavengers, antioxi- dants, anti-hormonal effects, cell growth inhibition, programmed cell death – all are terms that have been connected with the prevention of cancer by drugs or nutritional factors over the last thirty years. For the last ten years or so, the focus has been turning to a new field: epigenetics. Researchers working in epigenetics study the heritable effects on gene expression that occur independently of changes in the DNA sequence. Epigenetic mechanisms play a pivotal role in embryonic development, in tissue-specific gene expression and the memory formation, to name just a few examples, and enable the organism to adapt to changes in the environment. Disruptions to these processes contribute to ageing and the genesis of (chronic) disorders – also including carcinogenesis. Essentially, there are three epigenetic mechanisms that control gene expression (fig.1). Histone modification involves the attachment of small chemical methyl or acetyl groups to histones (cell nucleus proteins, around which the DNA is wound): this influences the packing of genetic material in the nucleus and plays both a positive and negative role in gene transcription. DNA itself can also be methylated: if this occurs in promoter regions not normally methylated, then expression of the gene is inhibited. Non-coding (micro-)RNAs, of which over 2,500 have been identified in recent years, are each thought to regulate several hundred genes and have more of a modulatory function. They affect the translation of messenger RNA (mRNA) into proteins, since they either influence mRNA stability or block protein synthesis by binding to the mRNA’s 3’ untranslated regions.
Fig. 1: Overview of gene expression regulation via epigenetic mechanisms. Histone modifications, DNA methylation and non-coding (micro)RNAs present attack vectors for natural substances sourced from nutrition.
Natural substances switch genes on The first pioneering research showing the potential influence of natural substances and nutritional factors on epigenetic mechanisms was completed in the US in 2003. A team from Rutgers University described how a constituent of green tea was able to inhibit DNA methyltransferases (DNMTs) and thus prevent the deactivation of tumour suppressor genes in a laboratory study. DNMTs are the proteins that attach the chemical switches to the DNA, thus making these less accessible for transcription factors. These initial findings were followed by a large number of other studies reporting on the capabilities of natural substances as methylation inhibitors. These include polyphenols from apple juice, coffee, soya beans and curry powder, selenium, vitamins, and sulphur-rich constituents in onions, garlic and brassicas (fig.2). Cell culture experiments have shown that these nutritional constituents inhibit promoter methylation, thus enabling the reactivation of a multitude of genes that are silenced during carcinogenesis. These genes play a key role in detoxification processes, in DNA repair and during cell differentiation, as well as influencing uncontrolled cell growth via regulation of the cell cycle and the induction of apoptosis. With hindsight, however, one must concede that the inhibitory potential of certain substances was overestimated – probably as a result of the methods used for the detection of changes in methylation – and that some of the results of earlier experiments cannot be reproduced by using modern quantitative methods. Furthermore, since most of the studies were in vitro experiments designed with the intention of analysing a few DNA regions at most, they do not offer a genome-wide view of these substances’ effects on DNA methylation.
Fig. 2: Inhibition of cancer-associated DNA methylation. In cell culture experiments, constituents from nutritional components, vitamins and trace elements such as selenium inhibit the activity or expression of DNA methyltransferases. As a result, silenced genes performing key functions in cellular processes such as detoxification, cell growth control or DNA repair are reactivated and contribute towards cancer prevention.
Information is generally scarce concerning the impact of nutritional factors on the methylation pattern in animal models or in human pilot studies. One exception is the research being conducted by Gary Stoner at Ohio State University (USA). For over 20 years, Stoner has been investigating the anticarcinogenic properties of black raspberry, a raspberry variety that is especially popular and widespread in the USA. In one study involving patients with colorectal cancer who consumed 45?g of freeze-dried black raspberry daily for up to nine weeks, silenced inhibitor proteins of the Wnt signalling pathway – often disrupted and permanently activated during colorectal cancer development – were reactivated, thus retarding cell growth. These findings were subsequently confirmed in rodent models for genetic or chemically-induced ulcerative colitis. The effect is ascribed to the anthocyanin pigments, which are also to be found in other blue and red fruit, including blueberries, cherries and grapes. Retarding the inflammatory response In 2004, researchers at the Linus Pauling Institute in Oregon (USA) were the first to show that a metabolic end product of the broccoli constituent sulforaphane inhibits the activity of histone deacetylases. These proteins are responsible for removing acetyl groups from histones. As a result, key proteins that control the cell cycle and programmed cell death can be reactivated, and the growth of cancer cells can be suppressed, as demonstrated both in vitro and in rodent tumour models. Activities similar to sulforaphane are seen with garlic constituents and the short-chain fatty acid butyrate, which is produced in high concentrations in the gut by the bacterial fermentation of dietary fibre. Another series of natural substances – including curcumin (from curry powder), anacardic acid (from cashew nuts), delphinidin (from pomegranate) and catechin (from green tea) – inhibits the histone acetyl transferase P300. P300 is an enzyme that transfers acetyl groups not only to histones but also to non-histone proteins such as the tumour suppressor protein P53, the androgen receptor or the transcription factor NF-kB. This could work to retard the hormone-mediated growth of prostate cancer cells and inflammatory responses, for example. miRNAs as biomarkers microRNAs (miRNAs) – small, non-coding RNA molecules – block messenger RNA transcription or influence their stability, thus regulating its translation into proteins. Since they are highly stable and can be detected in blood or urine, their suitability as biomarkers for the development or progression of diseases is also being investigated. The expression of some miRNAs in tumour genes increases during carcinogenesis. These “oncomiRs” – such as miR-21 – inhibit tumour suppression genes, promote tumour growth and inhibit apoptosis. Equally, tumour suppressor miRNAs such as let7 or miR-200 are often deactivated during carcinogenesis. This promotes the propagation of cancer cells, the formation of metastases and – importantly – the development of therapeutic resistance. In a series of cell culture experiments, a research team at Wayne State University (USA) showed that curcumin – and a chemically-modified form of curcumin in particular – was capable of normalizing the expression of these miRNAs and thus counteracting these processes (fig.3).
Fig. 3: Influence of curcumin on microRNAs. Curcumin and a synthetic curcumin analogue inhibit onco-miRNAs and activate tumour suppressor miRNAs. As shown by in vitro and animal model studies, not only is cell growth retarded, but genes are suppressed that participate in the development of therapy resistance, migration of cancer cells and the development of metastases.
Interestingly, miRNAs are also affected by tobacco smoke. In a study by the University of Genoa (Italy) conducted in 2010, rats were exposed to tobacco smoke daily for four weeks. Subsequent examination of lung tissue using microarray analysis demonstrated that, of 488 miRNAs investigated, around 50% were less strongly expressed in the animals exposed to tobacco smoke than in the control group. It was shown that the influence of tobacco smoke on miRNAs was reduced in animals that had received broccoli/watercress constituents or chemical substances promoting the detoxification of tobacco smoke carcinogens for three days prior to tobacco smoke treatment. Outlook As these few examples show, nutriepigenetics – i.e. the influence of nutritional components on epigenetic mechanisms – offers an interesting new approach for researchers working in cancer prevention. To date, available data are generally limited to in vitro investigations: few human studies are available that substantiate the functional relevance of epigenetic mechanisms for the cancer prevention efficacy of natural substances. Future research will need to identify best strategies for chemopreventive intervention targeting the epigenome. Bibliography
Huang J., Plass C., Gerhauser C. (2011) Cancer chemoprevention Photo: © istockphoto.com| Kudryashka |
L&M int. 4 / 2014
Free download here: download here The Author: |