|
Metal-organic frameworks: record-breakers in porosity
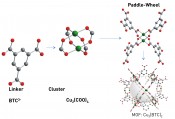
Metal-organic frameworks: record-breakers in porosityThe search for endless emptinessIn recent years, metal-organic frameworks have been setting one record after another in relation to specific surface area, with over 7,000m2/g now being achievable. Their modular construction and the large number of functionalities that can be built into the lattice make them both interesting and highly promising for a wide variety of applications. Metal-organic frameworks: Lego building blocks for inorganic chemists The structure of metal-organic frameworks (MOFs) is deceptively simple: one simply reacts a di- or tricarboxylic acid – such as terephthalic acid – with transition metal salts such as zinc or copper nitrate. In the solution, inorganic clusters then form such as Zn4O clusters or Cu2(OOC-)4 paddle-wheel units (Fig. 1) [1–3], which function as nodes and position the carboxylic acid molecules at the corners of highly symmetrical polyhedra (octahedra, cubes). Due to the bi- or trifunctional structure of the carboxylic acids (linker molecules), the clusters can now be cross-linked endlessly in three dimensions. This structural principle, a pioneering discovery made by Omar Yaghi, Susumu Kitagawa and Gérard Férey, has resulted in a multitude of new compounds. These compounds exhibit extremely high porosity, tailor-made pore sizes and functional groups on the inner surface, which can be utilized by a wide variety of applications [4]. Today, over 10,000 metal-organic frameworks are now known. The current record is now approx. 7,000m2/g for the inner surface (BET) – comfortably exceeding the specific surface areas achieved for conventional adsorbents (activated charcoal and zeoliths). The first materials were synthesized at the end of the 1990s. Today, we can now say that the number of metal-organic frameworks has virtually exploded. After about 15 years of academic research, the first potential applications are now starting to take shape in a wide variety of fields, including energy storage, gas separation techniques, and catalysis, etc. A number of startups have also been formed in the meantime: these are now offering metal-organic frameworks for research purposes or as samples for industrial use.
Fig.1: Modular structure of MOFs using Cu3(BTC)2 as an example
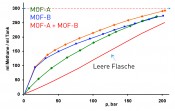
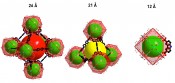
Applications in energy storage The use of MOFs in energy storage systems for gases such as hydrogen and methane was postulated at an early stage [5]. Since hydrogen can be stored only at low temperatures (-195°C), however, MOF-based hydrogen storage remains a long-term research topic. In contrast, methane (natural gas) can be stored at room temperature and pressures between 30 and 100bar [6–10]. This offers significant advantages in terms of capacity: by using a MOF-based storage system, up to three times the volume of methane can be stored in comparison to an empty compressed gas cylinder (Fig. 2). One such MOF, DUT-49, is currently one of the record holders in terms of gravimetric methane storage capacity (Fig. 3) [7]. Despite this, numerous challenges must still be met in turning this kind of MOF storage into marketable systems. As one example, filling generates large quantities of heat (enthalpy of adsorption) that need to be dissipated. Here, suitable thermal management models need to be developed. Volume markets are also difficult starting markets for new materials, due to intense price pressure. BASF is currently pioneering this commercialization work, having developed prototype vehicles that use the kinds of MOF-based natural gas systems described above. In this context, markets that envisage a distribution structure at the medial pressure range (60bar) are especially attractive. The market for natural gas-powered vehicles is also gaining ground in the US, where shale gas production has resulted in low gas prices.
Fig.2: Methane charge curve for MOF-filled fuel bottles (at 20 °C)
Fig.3: Multimodal pore structure in DUT-49 (DUT = Dresden University of Technology), with pore size specified
Moreover, the large volumes of heat generated by adsorption processes can also be used for latent heat storage. Such systems are currently being deployed in air-conditioning units and heat pumps (Fig. 4) [11–13]. Although these applications typically use zeoliths and SiO2 materials, MOFs are also ideally suited for such uses, due to their high water adsorption capacity. In addition, the partial pressure (relative air humidity) at which the MOF stores or releases water can be regulated in a flexible manner by configuring the pore sizes and integrating hydrophobic groups on the inner surface. This can be used for the effective optimization of energy needs.
Fig.4: MOF-coated heat exchanger Picture: DECHEMA MOF Roadmap
Gas scrubbing and separation The modular configuration of pore size and functional groups is a key benefit offered by MOFs. This approach can be used to remove certain molecules very selectively from mixtures of gases [14]. Two good examples include the removal of sulfurous compounds (e.g. thiophene) from fuels or hydrogen sulfide from air [15, 16]. Such separation techniques are important not only in the purification of fuels and scrubbing of breathable air but also for industrial processes. The use of MOF materials has even been proposed for the implementation of more demanding industrial separation techniques such as propane/propene separation – a task that consumes vast amounts of energy as a distillation process. Initial work here has shown that certain materials are in principle suited for use in implementing such separation processes, due to variations in the speeds at which they absorb propene and propane [17–19]. MOFs could also be deployed in gas filters used for scrubbing toxic gases. This involves integrating MOFs into textiles – by electrospinning, for example (Fig. 5).
Fig.5: MOF integration into fibers using electrospinning
MOF sensors and catalysis MOFs are highly suited for integration into sensors, since their properties – such as color on molecule absorption, or even the actual weight change – can be modified in predefined ways [20, 21]. Thanks to these qualities, they can be used to monitor room air or can even support long-term humidity monitoring within an industrial environment. Research teams have been deploying MOFs as catalysts for a long time: the possibility of anchoring a wide variety of materials in their hierarchical structures is paving the way for numerous potential applications in catalysis. Details of rhodium-based MOFs have now been published [22], which are suitable for hydrogenation. Palladium-based MOFs also exist. Notwithstanding such clear-cut applications, it can still be said that our understanding of the catalytic activity is modest at best. Securing a real grasp of this activity – and thus the targeted use of catalytically active centers in MOFs – can be achieved only if the defect chemistry of these materials can be understood and utilized. Accordingly, we may assume not only that numerous defects are present in MOFs on account of missing metal centers, but that metal centers can be formed that exhibit deviations from the ideal geometry. One problem arises from the fact that catalytically active metals often change their coordination sphere during a catalysis cycle. This is not possible if they are part of a rigid network. Yet if they change their coordination, the network collapses. This inherent problem again shows that catalytically-active centers can be installed only as defective imperfections or foreign atoms. An alternative approach is to use the functional groups of linkers to coordinate other metals. This model has been used to generate highly-selective and also highly active enantioselective catalysts [23]. Today, the scaling-up of MOFs can be considered unproblematic. At the turn of the millennium, milligram quantities of MOFs were being synthesized as single crystals (Fig. 6). Contemporary processes can synthesize many MOFs at the 10kg scale while also allowing geometry to be specified (spheres, monoliths, etc.) [24]. While prices are still relatively high due to the small volumes of materials produced, it is foreseeable that the price can be brought under EUR 100/kg once synthesis processes are being performed on a larger scale.
Fig.6: Scaling-up of MOF production in Dresden up to the 10?kg scale
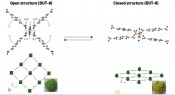
MOF switches The most fascinating property exhibited by MOFs, however, is that their porosity is also switchable [25]. Accordingly, a range of materials are available with the ability to open or close their pores at certain pressures. These are also termed “breathing” MOFs: as it “breathes”, all of the elementary cells in a MOF switch simultaneously from a porous, open structure to a closed structure or vice versa (Fig.7). This process means we can start with a material that is non-porous and end up with a material that has a specific surface area of several 1,000 m2 per g/material. These switching operations are triggered by small molecules at a specific partial pressure. This pressure-induced switching behavior is unique and is also referred to MOF “gating” [25, 26].
Fig.7: Switchable MOFs can reversibly change their structure from a closed to an open state.
Outlook The world of MOFs is currently seeing exponential growth. While work in initial years concentrated on MOF synthesis and fundamental properties, system integration and product development are now the strongest trends in the field. Recent work has focused on integration with electronic systems [27, 28]. A new German Research Foundation priority program was approved for this topic in 2015. This will also require greater efforts to be made in illuminating band structures, charge transport mechanisms and magnetic properties. And the modular structure has also inspired organic and polymer chemists to search for new approaches to constructing metal-free three-dimensional networks using the “Lego” principle. The search for new porous materials and their properties seems to know no bounds. There is still much for us to discover!
Bibliography Picture: © istockphoto.com| Vladimir Arndt |
L&M int. 4 / 2015
Free download here: download here The Author:Read more articles online |