|
Scientist
>
Dr Neha Chauhan
>
The critical role of fatty acid channeling between membrane and storage lipids
The critical role of fatty acid channeling between membrane and storage lipidsWhen lipid metabolism breaks downAlthough as thin as the skin of a soap bubble, they are nonetheless vitally important. Biological membranes, 10,000 times thinner than a human hair, form a physiological barrier to all the cells in our body and are thus responsible for mediating exchanges between the cell interior and the external environment. Minor changes to their composition, such as those involved in lipid metabolism disorders, can have fatal consequences for events within the cell and ultimately be the cause of serious illnesses. Membrane and storage lipids: a close physiological relationship The basic structure of all membranes is formed out of a hugely complex mixture of various lipids (from the Greek /lipos = fat), a class of substances of which cholesterol and lecithin are two of the more familiar members. Biological membranes form the cell’s barrier against the environment, thus ensuring that the cellular microcosm remains constant even under changing environmental conditions. In addition, numerous organelles with special-purpose functions within the cell are also enclosed by membranes in more highly-developed cells. Examples include the double nuclear membrane separating the genetic information (in the form of DNA) from cytosol or the cells’ “power plants”, the mitochondria, which are also enclosed in a double membrane. Other organelles possessing membranes include the endoplasmic reticulum or peroxisomes, for example. This intracellular compartmentalisation ensures that metabolic pathways for synthesis and degradation can exist side-by-side – separated by membranes – as precisely regulated processes. Alongside the membrane-forming lipids, it is the proteins embedded in these membranes in particular that perform specialised functions and whose activity is strongly influenced by the membrane’s lipid structure. These functions include (selective) substance transport through the membrane, signal functions and numerous biological reactions that are catalysed by enzymes within the membrane. In contrast to the membrane lipids, storage lipids – which occasionally make an unwelcome appearance at the belly and hips – are used primarily as energy reserves once other resources such as sugar are exhausted. Muscles in particular are reliant on the production of energy by the oxidation of fatty acids mobilised from storage lipids. Another key function performed by storage lipids is that of buffering a potential overflow of (free) fatty acids, so as to avoid the resultant toxic burden on the cell. Induced by fatty acids, this “lipotoxicity” is now being considered as a cause of lipid-associated disorders such as metabolic syndrome, obesity and diabetes mellitus type 2 [1]. The genesis of cancers is also increasingly being discussed in the context of lipid metabolism disorders. Researching the causes of lipotoxic alterations therefore forms one of the goals of research activities worldwide, so as to achieve a better understanding of the underlying mechanisms that govern the regulation of fatty acid uptake and its metabolism (see also the special research project LIPOTOX http://lipotox.uni-graz.at). Membrane-forming lipids and storage lipids are constructed from common precursors, including fatty acids. Some may be sourced from nutrition (examples include saturated fatty acids, trans-fatty acids, omega-3 fatty acids) while some may be produced by cells and tissues themselves as needed. Accordingly, this use of common precursors (fig.1) requires a sophisticated system of cellular regulation so as to ensure that membrane lipids are produced in the right kinds of qualities and the right kinds of quantities [2]. This is of fundamental importance for cellular growth in particular: every time the cell divides, the cellular membranes must also be duplicated accordingly. Research has already clearly demonstrated that the incorrect synthesis of storage lipids leads to a dramatic sensitivity of cells towards fatty acid excess [3]. On the other hand, the incorrect degradation of storage lipids itself results in impaired cell growth, since membrane lipid synthesis is thereby affected [4].
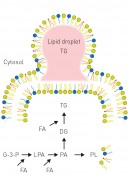
Fig. 1: Bottom: The formation of triglycerides (TG) and membrane phospholipids utilises the same types of precursors – lysophosphatidic acid (LPA) and phosphatidic acid (PA). Depending on fatty acid (FA) availability, a smaller or greater amount of triglycerides is formed, while membrane phospholipids (PL) are maintained at a constant level. If the biosynthesis of TG becomes disrupted, an excess of fatty acids causes changes in the membrane lipids and, in consequence, membrane malfunction and cell death. G-3-P: glycerol-3-phosphate; LPA: lysophosphatidic acid; PA: phosphatidic acid; DG: diglyceride; TG: triglyceride; PL: phospholipid, FA: fatty acids.
Top: Triglycerides (TG) are stored in structures termed “lipid droplets” (LDs). The biosynthetic mechanism for LDs are hotly debated: the current model assumes that TG accumulate between the leaflets of the membrane and that LDs “bud off” into the cytosol once they have reached a certain size.
Yeast, metabolic syndrome and cancer: an authentic experimental system for researching lifestyle diseases The enormous complexity of lipid metabolism, involving the participation of a great many organs and cell types, is an especial challenge for biomedical research. In research, simpler model systems are therefore used, such as fruit flies, nematode worms or various singled-celled fungi (yeasts). For lab experiments, these not only offer a more multifaceted approach but are considerably easier to handle while also being ethically sound. Alongside the well-known technological applications of (brewer’s) yeast in brewing and wine-/bread-making – and also, in recent years, in the fuel industry (bioethanol) – this organism has also established itself as an excellent model system for basic cellular research [5]. In recent years, for example, numerous Nobel prizes have been collected by scientists working in yeast research for the fundamental biological insights thereby achieved, which also have relevance for human cells (2001: Lee Hartwell, Paul Nurse – cell cycle; 2006 Roger Kornberg – transcription; 2009: Elizabeth Blackburn, Carol Greider, Jack Szostak – telomeres; 2013: Randy Schekman – vesicular transport). Yeast is also being successfully deployed in gerontology research and for the investigation of neurodegenerative diseases. Of the 6,000 yeast genes known, around one third have a counterpart in the human genome, while some 20% of genes that are associated with genetic diseases in humans have a homologue in yeast. Newly-developed pharmaceuticals are routinely tested on yeast cells, a process that simplifies the identification of molecular targets. Furthermore, a highly diverse portfolio of technologies is available not only for the investigation of individual factors but for the systematic study of the cell across the totality of its physiological processes. In the lab, the cells’ doubling time of just 90 minutes makes them easy to cultivate in large quantities while facilitating biochemical and genetic manipulation. This short doubling time is all the more astonishing when one considers the cells’ highly complex structure, which resembles that of human cells. Alongside the membrane-enclosed cell nucleus, yeast possesses mitochondria, the highly branched membrane system of the endoplasmic reticulum, vesicles, vacuoles and – as a physiological barrier to the outside world – a plasma membrane (fig.2). As with cells of plant and animal origin, lipid droplets are also present, of the type responsible for lipid storage (fig.3).
Fig. 2: Subcellular organelles in yeast. The membranes in question are made visible under the fluorescence microscope by staining with specific dyes. The cell size is 5–7 µm. Top row, from left to right: vacuoles, endoplasmic reticulum, mitochondria. Bottom row: cell nucleus, lipid droplet, plasma membrane.
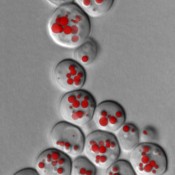
Fig. 3: Fluorescence microscopy image of lipid droplets (LD) in yeast cells. LD are stained using specific fluorescent dyes and can then be made visible under the microscope. LD size and quantity provides information about possible disruptions to lipid metabolism. This yeast culture lacks the two essential lipases that are responsible for the breakdown of triglycerides.
Yeast and cancer cells are especially similar in terms of lipid metabolism [6], which underlines the strength of the yeast system as a model. The primary enzyme involved in human fat breakdown – adipose triglyceride lipase – also has a counterpart in yeast [7]. Disruption to fat breakdown in yeast is accompanied by the development of “obese” yeast cells, which deposit the excess storage lipid (TG) in lipid droplets. The degradation of storage lipids is necessary in order to supply precursors for the synthesis of membrane lipids during cell growth: if enzymes required for the breakdown process are absent, cells not only become fat but also exhibit significant retardation of cell division. Regulatory factors in the cell cycle programme that respond to altered lipid mobilisation are now a major focus of research [8]. If cells lack the ability to respective fat – perhaps due to the deactivation of genes required for fat synthesis in the respective mutants – cells develop a highly sensitive response to an excessive surplus of fatty acids and die off rapidly since the membrane equilibrium is markedly disrupted. This underlines the importance of storage lipids as a “buffer”, so as to intercept a surplus of fatty acids – taken up via nutrition, for example – and to render this surfeit harmless for the organism. A malfunction within the fatty acid detoxification process could well be the causative agent of damage to the b cells in the pancreas responsible for insulin production and thus contribute to the genesis of type 2 diabetes in obese patients. The lipid droplet: the unknown organelle Until a few years ago, the general opinion was that storage lipids were stored within the cell as inert droplets of fat. Yet this fat droplet is actually a highly complex organelle: lipid droplets (LDs) are enclosed by a membrane lipid layer within the cell (fig.1), while their surfaces are adorned with a wide variety of proteins [9]. These proteins are used in the synthesis and degradation of storage lipids (or the regulation of such processes) and have a key role to play in interaction with other organelles – such as mitochondria. One result of storage lipid cleavage is the oxidation of the released fatty acids in the mitochondria, for example. In humans and animals, the fatty acids released from adipose tissue are transported via the bloodstream to heart and muscle tissue or the liver, where they are oxidised or converted back into storage lipids. According to the model currently under discussion, triglyceride is synthesised in the membrane of the endoplasmic reticulum and stored between the two membrane halves; on reaching a certain size, the developing LD then “buds off” into the cytosol. This model can be used to explain the surface structure of the LDs, which are enclosed by a phospholipid monolayer – unlike all other organelles, which feature a double layer (see fig.1). Research to date has identified hundreds of proteins associated with lipid droplets, and which potentially play a role in the biogenesis of these organelles or in lipid storage and mobilisation. Although the biogenesis of lipid droplets plays a major part in the storage of fat, a number of fundamental questions remain unanswered. Is the prevailing model – i.e. TG storage between the membrane halves – actually correct? When and how does “budding off” occur? How do LD-associated proteins reach the organelle – and how are these processes regulated? How is the synthesis of membrane lipids and storage lipids regulated? The close physiological interrelation between membrane phospholipids and triglyceride storage/mobilisation, along with their associated disorders, are considered to be multiply involved in the genesis of our “lifestyle diseases” and now form a major branch of global biomedical research. One may safely assume that the field of yeast research will continue to make significant contributions to our understanding of lipid homeostasis in human cells.
Bibliography Picture: © istockphoto.com | Ugreen |
L&M int. 1 / 2015
Free download here: download here The Authors:Read more articles online |