|
Complex API delivery
Complex API deliveryCellular transport proteins and API transportA medicine's potency often depends on the concentration of its active ingredient (API) at the target site. Medicines are usually delivered remotely to this target site, however. The API must first dissolve and traverse local barriers such as the intestinal wall before it can enter the bloodstream and then reach its target site. For a long time, work in this area was guided by the dogma that API diffusion was the force driving absorption by the body or the cell. Ideally, the API should bear no charge and should be lipophilic, so as to diffuse readily through membranes. Today, we now know that the majority of active ingredients – and indeed many nutritional components – are transported across membranes in an ionised form by transport proteins. Transport proteins are proteins integral to a cell membrane that actively or passively mediate the permeation of a substance across the membrane. Unlike free diffusion, transport-mediated permeation has characteristics of both saturation and inhibition, and can occur both passively along a concentration gradient as well as actively against a concentration gradient by utilising energy. This energy can be consumed either directly by the transport protein itself (primary active transporter) or by the indirect coupling of a transporter with a primary active protein (e.g. an ATPase). We distinguish between uniport systems, which transport substrate molecules in one direction, symport or cotransport systems, which simultaneously transport two or three separate substances in the same direction, and antiport systems, which transport two or three substances in opposite directions (fig. 1). Other components include ion pumps (e.g. Na+/K+ ATPases), ion channels and water-transporting aquaporins. The decoding of the genome has led to an internationally binding classification of transport proteins and ion channels by the International Union for Biochemistry and Molecular Biology (IUBMB) in the form of the “Transporter Classification Database” (TCDB), which lists over 600 families of transport proteins. The human transport proteins encompass the superfamilies of the annexins, ATPases, calcium channels, potassium channels, sodium channels, ATP-bin ding transport proteins and the human solute carriers (SLCs). A list of all genes that code for these proteins can be found on the web page of the HUGO Gene Nomenclature Committee (HGNC) (www. genenames.org/ gene families). Today, we estimate that approximately 10% (~2,000) of all human genes code for transporters, while the human solute carriers currently encompass 52 transporter families with 395 genes (www.bioparadigms. org/slc/intro.htm). The nomenclature of this protein group was introduced in 1990 by M. Hediger, who first cloned the Na+/ glucose cotransporter in E. Wright's research team in 1987 [3]. As a rule, a transporter is assigned to a specific family if at least 20% of its amino acid sequence is identical with that of the other members within the family. The SLC protein and ATP-binding superfamilies are of particular interest, since they participate in the resorption, distribution and excretion of many pharmaceuticals, thus determining their bioavailability. The recognition of candidate compounds by these transport proteins is now routinely investigated during the development and licensing of new APIs, and has led to the publication of corresponding regulatory guidelines by the FDA and EMA. Drug interactions at the transporter level Numerous drug substances are capable of inhibiting transport proteins and consequently modifying the pharmacokinetics of other transport substrates. In some cases, this has even led to the drug's withdrawal from the market. One noted example is cerivastatin, an HMG-CoA reductase inhibitor, whose ingestion has led to fatal myotoxicity in a number of cases. An analysis showed that some patients had simultaneously been taking the antihyperlipidemic agent gemfibrozil, resulting in as much as a 4.4x increase in the plasma concentrations of cerivastatin [1]. The root cause was an inhibition of CYP2C8-mediated metabolism and an inhibition of the hepatic absorption of cerivastatin by gemfibrozil glucuronide as mediated by OATP2 (organic anion transporting protein 2, OATP2/OATP1 B1:- SLC21A6)) [2]. To prevent repeat occurrences of such cases, the FDA issued the guideline “Drug Interaction Studies, Study Design, Data Analysis, Implications for Dosing, and Labelling Recommendations” in 2012. In the same year, the EMA finalised its “Guideline on the Investigation of Drug Interactions”, which devoted particular attention to the discussion of transportermediated drug-drug interactions. ATP-binding proteins A characteristic common to the ABC (ATP binding cassette) transporter superfamily is its capacity for binding and hydrolysing ATP, and thus obtaining the energy required for substrate translocation. ABC transporters act unidirectionally either as import or export proteins and can be found in both simple and complex organisms. While ABC export proteins are expressed by both prokaryotes and eukaryotes, ABC import proteins have previously been detected exclusively in prokaryotes. With an estimated 80 systems, ABC transporters constitute the most extensive protein family in E. coli and represent about 5 % of the bacterial genome. In humans, 48 ABC transporters have now been described in seven families. In modern nomenclature, the seven gene families coding for these transporters are termed ABCA to ABCG. These transporters are involved in lipid and cholesterol transport, in antigen presentation, in mitochondrial iron homeostasis, in ATP-dependent regulation of ion channels and in resistance to chemotherapeutical agents. The ABC export pumps P-glycoprotein (MDR1, P-GP; BCB1) and breast cancer resistance protein (ABCG2) have especial significance at the blood-brain barrier, where they form a key component of the barrier for the protection of the central nervous system. Unfortunately, this means many CNS disorders are difficult to treat, since many potentially powerful active ingredients therefore cannot pass into the brain. This becomes especially clear in the process of treating brain tumours or metastases, where the use of cytostatic drugs is generally ineffective. As one example, the mitotic inhibitor paclitaxel (Taxol), used successfully in the treatment of breast and ovarian cancers, cannot be deployed for glioblastomas, since it is a P-glycoprotein substrate and is therefore unable to traverse the bloodbrain barrier. Animal experiments in rats with implanted human glioblastomas have impressively demonstrated that the inhibition of P-glycoprotein in the blood-brain barrier by simultaneous administration of a blocker can lead to a drastic elevation of paclitaxel concentration in the brain (fig.2) and, consequently, to a significant reduction in tumour volume [4]. Transport proteins and disease Transport proteins may also be directly involved in disease, and transporter defects may also be the cause of illness. The glucose transporter SLC2A2 (GLUT-2) is expressed predominantly in the liver, pancreas, small intestine and kidneys, and enables insulindependent glucose transport. Mutations in the SLC2A2 gene cause the rare Fanconi- Bickel syndrome, a glycogen storage disease. The glutamate transporters SLC1A2 and SLC1A3 are also suspected of pathogenic involvement in amyotrophic lateral sclerosis, Alzheimer's, autism and schizophrenia. Members of the family of SLC13 transporters (Na+-coupled di-/tricarboxylate/ sulphate transporters) – namely SLC13A2 (NaDC1) and SLC13A3 (NaDC3) – are also suspected of involvement in the formation of kidney stones and the pathogenesis of the metabolic disorders glutaric acidemia type 2 and Canavan disease [5]. The anion transporter SLC26A4 is ascribed a role in deafness, SLC31A1 and SLC31A2 a role in the copper storage disorder Wilson’s disease. One of the most well-studied genetic diseases is cystic fibrosis (also known as mucoviscidosis), whose cause is ascribed to mutations on the long arm of chromosome 7. Cystic fibrosis is the second most common congenital metabolic disease for lightskinned populations, in which disease incidence is around 1 in 2,000 newborns. The affected gene codes for CFTR (Cystic Fibrosis Transmembrane Conductance Regulator (ABCC7)), a chloride channel that is a member of the ABC transporters. Disruptions to this channel mean that secretions produced by the bronchia, pancreas, liver and small intestine do not contain enough water: these then become viscous, resulting in a wide range of malfunctions in the affected organs. To date, over 1,000 separate mutations are known for the CFTR gene. The commonest mutation for this gene, ?F508, causes a deletion of phenylalanine at position 508 in the protein and is present in seven of ten people with cystic fibrosis. Another – although much rarer – hereditary disorder that can be traced to an ABC transporter defect is Tangier disease. This is a lipid metabolism disorder whose underlying cause is a defect in the ABCA1 gene that codes for a cholesterol transporter. This results in reduced formation of high-density lipoproteins and elevated levels of cholesterol storage.
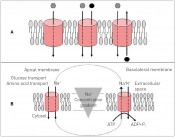
Fig. 1: A) Unidirectional transport, cotransport, antiport system. B) Secondary active transport: coupling of an Na+ amino acid or glucose transporter in an apical membrane to an Na/K ATPase in the basolateral membrane. The energy necessary is provided by hydrolysis of ATP by the ATPase (from "Biopharmacy", VCH-Wiley; with permission of the publisher).
Fig. 2: A) Accumulation of the Pglycoprotein substrate paclitaxel in the brain before and after peroral administration of the P-glycoprotein inhibitor valspodar (PSC-833). B) Glioblastoma size 35 days after tumour implantation in control animals, after treatment with paclitaxel alone and after peroral administration with the P-glycoprotein inhibitor valspodar shortly before IV administration of paclitaxel.
More well-known is the autosomal recessive Dubin-Johnson syndrome, which arises from a mutation in the gene for multidrug resistance-related protein 2 (MRP2, ABCC2). The protein causes ATP-modulated secretion of glucuronidated (conjugated) bilirubin to the gallbladder by the liver. If the protein is non-functional, then bilirubin cannot be transported into the gallbladder capillaries: this leads to accumulation in the liver and to a backflow of conjugated bilirubin into the blood. Fortunately, prognosis is good for the disease and treatment is usually not necessary. Mutations in the ABCB1 gene, which codes for the best-characterised ABC transporter P-glycoprotein (ABCB1), are believed to increase susceptibility to a sub-type of inflammatory bowel disease (inflammatory bowel disease 13). Quite possibly, the export pump is also involved alongside ABCG2 (breast cancer resistant protein, BCRP), ABCC1 (multidrug resistance-related protein 1, MRP1) and the cholesterol transporter ABCA1 in the pathogenesis of Alzheimer’s disease, since these proteins help in the clearance of amyloid-ß from the brain. Isolated cases are also known from veterinary medicine. Collies, shelties and bobtails must not be treated with the antiparasitic agent ivermectin, since defects in the ABCB1 gene have often been observed in these dog breeds. As a P-glycoprotein substrate [6], the neurotoxin ivermectin can traverse the bloodbrain barrier in dogs lacking the P-glycoprotein function and lead to the death of the animals so treated. On account of its formidable role in the body’s defences against toxic substances and the development of resistance to chemotherapy, P-glycoprotein has often been the target of recent experiments that have attempted to block the compound and thus also achieve clinical reversal of cytostatic resistance. Yet results to date have been disappointing. An interesting phenomenon is observed in the treatment of epilepsy. Therapeutic resistances are a frequent result of prolonged treatment with anti-epileptic drugs, probably due to an induction of ABC export proteins in the vicinity of epileptic foci, which in turn decreases the concentration of the anticonvulsant [7]. Even this handful of examples clearly illustrates the decisive role that transport proteins play in physiology, pathophysiology, toxicology and the effects of medications. While our knowledge of these proteins has increased immensely over the last two decades, many questions still remain unanswered. How does their recognition of substrates work, for example? Which signal cascades regulate them and how can findings about the structure of these proteins be used for rational drug design? Answering these questions will improve our understanding of the proteins’ physiological role, further illuminate the pathomechanisms of disease and help develop new strategies in drug discovery.
Bibliography Picture: © panthermedia.net | lightwise |
L&M int. 3 / 2014
Free download here: download here The Authors:Read more articles online |