|
The earliest plasma marker for myocardial infarction
The earliest plasma marker for myocardial infarctionFabulous FABPThe application of Fatty Acid-Binding Protein (FABP) as a plasma marker for the diagnosis of acute myocardial infarction was first suggested in 1988. Currently, FABP is proven to have added value for the diagnosis of patients presenting with chest pain suggestive of myocardial infarction, especially in the early hours after onset of symptoms. The routine application of FABP for this purpose not only will improve patient outcome but also markedly reduce costs for those cases in which an infarction can be excluded. This article describes the almost 25 years of fascinating history between discovery and commercial application. “Time saves heart muscle” Suspected heart attack? Rapid triaging of patients admitted to hospital with chest pain is needed to include positive cases for immediate application of appropriate therapy. An acute myocardial infarction (AMI) is caused by obstruction of one or more coronary arteries leading to lack of oxygen supply and ultimately dysfunction of that area of the heart. The sooner the arteries can be re-opened – by installing thrombolytic therapy – the less cardiac muscle will die and the better cardiac performance is maintained (“time saves muscle”). However, rapid triaging is equally important to exclude low-risk patients who can safely then be sent home. The latter not only adds to proper patient care but also to a marked reduction of costs for health care. AMI markers Biochemical plasma markers for myocardial injury have now for years been accepted as the primary tool for AMI diagnostics. These markers include creatine kinase isoenzyme MB (CK-MB), troponin T, and troponin I. The advantage of these markers is that an elevation of their plasma concentration, together with typical complaints of the patient, is almost absolute proof of the occurrence of an AMI. Changes in the electrocardiagram (typically, S-T segment elevation) may also be seen after an AMI but, importantly, some 25% of patients do not show such changes so that this parameter alone cannot be applied for making a final diagnosis. The biochemical markers, however, have the disadvantage that, following an AMI, their plasma concentration does not start to increase until about 4 (CK-MB) or about 6 hours (troponins) after onset of symptoms. As a result, for a significant number of patients presenting with chest pain their final diagnosis is delayed, while for saving lives in the early hours after onset of AMI symptoms speed is the key! The corollary is that the ideal biochemical marker for AMI would show a much more rapid appearance in plasma after cardiac injury. Both myoglobin and Fatty Acid-Binding Protein (FABP) have been found to be elevated in plasma within 2 hours after symptom onset. Clinical studies have revealed that of these, FABP performs more specifically than myoglobin, making FABP the preferred plasma marker for early diagnosis or exclusion of AMI. The discovery of a “smart dwarf”, FABP The cytoplasmic protein FABP functions as an intracellular fatty acid carrier in parenchymal cells, thus supplying essential substrates for energy production in the myocytes. It comprises as much as 1–2% of total cardiac cytosolic proteins, making it one of the most abundant cytosolic proteins. This is very good for a potential biomarker. It was as early as in 1988 that Jan Glatz suggested FABP as a plasma biomarker [1]. He was studying the biological function of FABP in the transport of long-chain fatty acids across the cellular cytoplasm. When performing experimental studies with the isolated perfused rat heart subjected to ischemia and reperfusion, he observed that FABP was released from damaged myocytes into the perfusion buffer. This serendipitous finding led him to suggest FABP as a potentially superior marker of myocardial injury in humans. It was reasoned by him that its relatively small size (14.5 kDa) and abundance in the heart could allow a rapid release of significant amounts of FABP from injured myocardium into the circulation. Subsequent mechanistic studies disclosed that these assumptions indeed were correct. Biochemical AMI markers compared Table 1 compares the characteristics of proteins that can serve as a plasma biomarker for AMI. It is clear that FABP stands out from the other marker proteins in that it is detectable in plasma within 1–2 h after myocardial injury, which is much earlier than any of the other marker proteins. In fact, some studies have reported its elevated presence in plasma as soon as 30 min after AMI onset. Although referred to as heart-type FABP, this protein – unlike CK-MB and the troponins – is not absolutely heart-specific but is expressed only in relatively low amounts in skeletal muscles. Peaks of FABP are reached at 6–8 hours, and the blood plasma level returns to normal within 24–36 hours due to rapid elimination by the kidneys. The latter is the ‘beauty’ of FABP: the rapid renal clearance keeps the normal (reference) plasma concentration very low so that upon release of FABP from cardiac muscle after AMI the upper reference level (established for groups of individuals) is soon surpassed, enabling a very early diagnosis. In contrast to this ‘dwarf protein’, all other marker proteins (except the small size muscle molecule myoglobin) are larger in size: troponin I (TnI) is 1.5-fold larger, troponin T (TnT) 3 times larger, and CK-MB 5 times larger. Myoglobin has the disadvantage that it is more abundant in skeletal muscle than in cardiac muscle (up to 3-fold higher content in skeletal muscle), resulting in a relatively high plasma reference concentration which hampers an early diagnosis. The troponins, cardiac TnI and cardiac TnT, are structurally bound proteins that upon AMI first must be dissociated from the myofibrillar structures before they can be released into the circulation. Therefore, their release from injured myocardium follows a different pattern with elevated plasma concentrations occurring from approximately 6 h to more than 1 week after infarction. Hence, the so-called diagnostic window of the various marker proteins differs significantly with FABP being the preferred early marker and cTnI/cTnT the preferred late marker (Fig. 1) [2,3]. A panel of marker proteins, e.g., FABP together with cTnI or with cTnT, would cover the entire range and allow the proper diagnosis of patients with chest pain from almost immediately until more than a week after AMI onset. FABP has a further diagnostic advantage in that due to the early normalisation of its plasma concentration after AMI, a re-infarction (second AMI) occurring within a few days from the first event can be monitored from a new rise of plasma FABP, which is not the case for CK-MB nor the troponins as these would just remain elevated.
Fig. 1 Release of FABP, myoglobin, CK-MB and cardiac troponins from the injured heart into plasma after acute myocardial infarction (AMI).
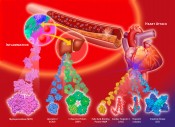
Fig. 2 Proteins that upon a myocardial infarction will be released into plasma (in the order in time from left to right). An early marker needs a rapid test Because FABP, unlike CK-MB, is a non-enzymatic protein, its detection and quantification must be performed with an immunochemical assay. A large number of immunoassays for FABP have been described and have been successfully applied for retrospective analyses of plasma FABP in patient samples. However, the implication of FABP for clinical decision-making in the case of suspected AMI would require tests with a performance time < 10 min. For immunoassays this represents quite a challenge. In June 1993, Jan Glatz presented his novel findings on FABP in the diagnosis of AMI for the first time to an international conference of the Belgian Society for Clinical Chemistry, held in Brugge, Belgium. He was approached by Dr. Hans-Georg Eisenwiener, head of the Research Division at Roche Diagnostics, Basel, Switzerland. In the same year they embarked on a collaborative project to develop a microparticle-enhanced turbidimetric assay to be performed on a conventional clinical chemistry analyzer (performance time 8 min) [4]. For this, specific monoclonal antibodies showing exceptionally high affinities for FABP were generated, and much attention was paid to standardization of measurements using recombinant human heart-type FABP produced under strictly controlled conditions. Both this turbidimetric assay and an extremely accurate and reproducible ELISA method for FABP have been instrumental to revealing the added value of plasma FABP as early biomarker for AMI diagnosis in a number of large trials. For instance, the European multicentre clinical trial EuroCardi, comprising 4 hospitals in 3 countries, revealed the superiority of FABP [5]. It unanimously revealed a significantly better performance of FABP over the other small biomarker, myoglobin, for early AMI detection as well as early estimation of infarction size. It also showed its advantages compared with the ‘slow’ troponins. At this time, the German company Boehringer Mannheim offered immunochemical tests for myoglobin, CK-MB and also a test for the new cardiac marker, troponine T. The troponine test was a milestone in cardiology, developed by Dr. Hugo Katus in Germany. In spring 1998, Roche and Boehringer Mannheim merged. Roche decided after this merger to concentrate on the troponine T test taken over from Boehringer Mannheim. This was understandable as the TnT test was well-appreciated and well-introduced to physicians internationally already and had been adopted as a ‘golden standard’ by cardiologists. Unfortunately, the cardiac marker division of the new company (based at the former Boehringer plant in Mannheim) did not appreciate the performance of FABP, especially the added value for early infarction diagnostics, whereby the combination of FABP and TnT could markedly extend the diagnostic window. The consequence was that the turbidimetric FABP test that had been developed with so much care was not launched to the market. This regrettable event also made other (smaller) biotech companies to reconsider their efforts to build an FABP test. Biosensor technology for FABP In the same period the Fraunhofer Institute of Chemical and Biosensors (ICB) in Münster had started a project to develop a biosensor for FABP. This project was supervised by Reinhard Renneberg who had moved in 1991, after German unification, from the Central Institute of Molecular Biology (Berlin-Buch) to the ICB to become head of the Department of Immunosensors. Like Jan Glatz, he immediately took an enthusiastic view of the new biomarker. His ICB-Department constructed an immunosensor-based instrument. This was the first bioelectrochemical approach worldwide to quantify FABP in plasma samples. Unfortunately, it could not work with full blood due to electrochemical interferences with red blood cells. “Crisis means danger plus chance” While the stage for FABP was far from promising, events developed internationally in different directions. This follows the Chinese character for “crisis” (Fig. 4) which wisely combines danger and chance. Reinhard Renneberg accepted a professorship at the Hong Kong University of Science and Technology (HKUST), currently (2012) ranking as the top Asian university. Together with Dr. John Sanderson of Prince of Wales Hospital and the small Chinese company EY labs Inc. he managed to get a government grant of 4.5 million HKD (450 k€) to develop a reliable and cheap full-blood FABP rapid test for the huge Chinese market. In parallel, in Berlin-Buch (Germany) Ilka Renneberg started in 2000 the first FABP company, 8sens biognostic Ltd., targeting Europe. Together with the producer and distributor rennesens GmbH, they produced a credit card style FABP rapid test [6]. The card needed three hanging drops of blood and it took 15 min to get a reliable YES or NO result. The design was great in style, however, but quite expensive in production. 8sens is currently marketing a follow-up test, a simpler cassette-type rapid test for FABP and a second test for troponin I. In this way an early and a late marker can be evaluated at the same time and be quantified with a reader.
More recently, in 2008 the small biotech company FABPulous was started in the Netherlands (www.fabpul
The Chinese character for ‘crisis’.
Several other companies now also offer FABP plasma tests. For instance, Randox Laboratories Ltd. has included FABP in their Biochip Array Technology panel which enables the simultaneous assessment of multiple AMI biomarkers in a single patient sample using the MultiStat rapid testing platform. Results are available within 30 min. In China, Reinhard Renneberg launched in 1995 a university-based biotech company, Renneberg & Caughterley Biogenius Ltd (R&C Biogenius) at HKUST. It was supported generously by the Small Company Development scheme (SERAP) of the Hong Kong government. R&C developed cassette style tests for FABP, troponin I, CRP and neopterin, licensed and transferred the technology across the border to KSB (Shenzhen Kang Sheng Bao Bio-Technology Co, Ltd), a producer company in Shenzen (PR China). KSB and R&C Biogenius in turn applied for a Sino-FDA approval in Beijing which was granted in 2009. This means: the FABP test is now available commercially in China [7]. A quantitative version of the FABP rapid test is available worldwide except in the USA through Concile GmbH (Germany, www.concile.de) and 8sens.biognostic (Germany, www.biogno FABP is more than a fabulous marker FABP is a sensitive marker that detects minor myocardial injury like that occurring in selected patients with unstable angina pectoris. In a prospective study comprising more than 1,400 patients it was shown that FABP predicts long-term mortality after acute coronary syndromes (ACS, which includes AMI and unstable angina pectoris) and identifies patients at risk for subsequent cardiovascular events [8]. Importantly, FABP is able to distinguish between low-risk and high-risk patients across the wide range of plasma troponin values and at all parts of the ACS spectrum, thus showing that FABP offers independent prognostic information [8]. Similarly, other studies have disclosed that in patients with congestive heart failure plasma FABP identifies those at high risk for future cardiac events. A new development in the field is the generation of high-sensitivity troponin assays (hsTnT and hsTnI). The limit of detection of these assays is about 10-fold lower than that of conventional assays. This suggests their application for diagnosing smaller AMIs otherwise undetected, or for identifying AMI earlier when abnormal troponin levels are too low for detection by conventional assays. While recent reports describe an improved performance of hsTnT when compared to conventional TnT, various studies have also demonstrated frequent elevation of plasma troponin in asymptomatic patients with stable coronary disease, pulmonary embolism, etc. These newer data challenge the application of hsTnT for triaging patients with acute coronary syndromes. Very recent studies have compared the performance of plasma FABP and hsTnT in the emergency room to conclude that FABP performs at least similarly, if not better, than hsTnT, and certainly earlier [9]. As a result, each of the biomarkers has its own characteristics, with FABP being the preferred marker to rule out AMI in the early hours after onset of symptoms and (hs)TnT or (hs)TnI the preferred marker from 3 hours onwards after presentation. The future is golden Despite the strong data available for FABP on its performance both as a biomarker for early triaging of patients with chest pain and as a prognostic marker for future cardiac events, the use of FABP is not yet widespread. We predict that this will change in the near future, when more POC tests and tests for clinical chemistry analyzers become available. FABP will then be adopted as an early plasma marker to be applied besides a late marker such as cTnT or cTnI. In emergency care diagnostics the future focus will be on exclusion of AMI, as this comprises some 80% of patients presenting with chest pain. FABP is excellently suited for this purpose, and in this way will enable a major reduction of costs otherwise spent on hospitalization of non-AMI patients. Given the large numbers of patients who present with chest pain (for instance, in Germany ca. 400,000 annually) it is clear that FABP is destined for becoming a new Golden Standard for life saving. Acknowledgement We would like to thank Dr. Hans-Georg Eisenwiener for continued support and stimulating discussions.
Bibliography Abbildung 1: Ming Fai Chow |
L&M int. 2 / 2013
Free download here: download here The Authors:Read more articles online |