Scientist
>
Dr Jochen Türk
>
Instrumental and effect-directed analysis in the oxidative degradation of micropollutants
Instrumental and effect-directed analysis in the oxidative degradation of micropollutants
Pharmaceuticals in wastewater
Occurance and fate of micropollutants, metabolites and transformation products in the water cycle is placing increasingly higher demands on instrumental analysis in terms of resolution and sensitivity. As yet, effect-directed analysis methods play a subordinate role in water analysis work. Yet for evaluating unknown samples or ingredients, these methods form a perfect complement to instrument-based identification. Indeed, in the detection of estrogenically active substances, bioanalysis is considerably more sensitive than mass spectrometry detection methods.

Rising concentrations of micropollutants in treatment plant effluent are placing increasing demands on wastewater treatment. Conventional facilities using standard technologies remove only a small portion – if any – of such substances from the wastewater. Elimination of such substances can be achieved by making use of advanced oxidation processes (ozonation, UV oxidation). The removal of hormonally active substances, pharmaceutically pharmaceuticals and personal care products (PPCP) and industrial chemicals from wastewater goes hand in hand with reducing the chemical oxygen demand (COD) and dissolved organic carbon (DOC) compounds. Key questions about the formation of toxicologically relevant transformation products (TPs) still remain unanswered, however. For the evaluation of oxidation products, cost-effective and sensitive detection methods are essential. Despite the availability of increasingly powerful GC-MS and LC-MS equipment, instrumental analysis as it currently stands is incapable of quantifying the environmental quality standard recommended by the European Union of 0.035ng/l for the synthetic hormone 17-ethinylestradiol [1]. Following enrichment, in vitro assays can be used to measure estrogenic activity as a sum parameter. As a reference point, a 17-ß estradiol equivalent quotient (EEQ) is selected. Biological analysis is unable to furnish statements about individual substances, however. This being so, instrumental and effect-directed forms of analysis supplement each other ideally as complementary methods.
Determining endocrine effects
Figure 1 shows the basic principles of the in vitro assay A-YES®_aqua 1.0 (new_diagnostics GmbH, Freising), an assay based on the transgenic yeast Arxula adeninivorans. The sum parameterisation of estrogenic activity is based on the binding of estrogenically active substances to the corresponding human receptor that is integrated into the yeast cell genome. The binding of estrogenically active substances to the receptor induces the expression of the phytase reporter enzyme, which is released into the medium (Figure 1a). The phytase is then detected photometrically, using an enzyme-substrate reaction (figures 1b and 1c). In this context, it was possible to demonstrate that the test system validated for mineral water is also suitable for testing treatment plant effluent. Without further concentration, the limits of detection achieved lay between 13 and 19ng/l EEQ. Following enrichment on an Oasis HLB solid phase (Waters, Eschborn), a limit of determination of 0.02ng/l was achieved [2]. Accordingly, the method is suitable for determining whether or not treatment plant effluent and surface waters comply with the recommended environmental quality standard.
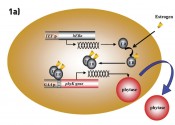
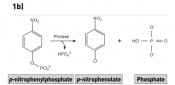

Fig. 1 Schematic representation of the basic A-YES mechanism. a) Binding of estrogenically active substances to the continuously expressed human estrogen receptor (ER) and subsequent formation of the phytase reporter enzyme (photo 1a: IPK Gatersleben). This reaction takes place during incubation for 24h at 30°C and 700–750 RPM. b) Enzyme-substrate reaction for detecting the phytase formed. The p-nitrophenolate formed produces a yellow colour on addition of the developer solution (NaOH). c) 96-well microtitre plate with yellow-coloured samples, measured in photometer at 405nm.
Online SPE-LC-MS/MS determination of fluoroquinolones
In the course of the projects presented here, an investigation was made of the chemical and toxicological evaluation of oxidative wastewater treatment techniques. In the context of drinking water treatment, estrogenic effects join cytotoxicity, genotoxicity and the mutagenic properties of unknown substances in the water cycle as the most worrying factors to consider.
As one example, the following discussion concentrates solely on the fluoroquinolones ciprofloxacin and ofloxacin, present at concentrations of between 30 and 200ng/l in WWTP effluent. Investigations of the toxicity of fluoroquinolones before and after oxidative treatment are of particular interest, since previous research has provided evidence that ciprofloxacin produces toxic effects [3, 4]. An online SPE-HPLC-MS/MS method has been developed for the rapid and sensitive analysis of ciprofloxacin and ofloxacin. Following the enrichment of 10 ml of the water sample on an HySphere C18HD SPE cartridge (Spark Holland), elution proceeds in HPD focusing mode with 200µl acetonitrile:water (40:60) injected within one minute into the HPLC flow of 300µl/min 5% acetonitrile in water with 0.1% formic acid. After one minute the solvent gradient starts with a flow of 500µl/min. To keep analysis times as rapid as possible and achieve a high level of sensitivity, the decision was made to skip baseline separation of the two fluoroquinolones; the system was re-equilibrated again after just 2.5minutes. If we look at the 4 MRM transitions shown in Figure 2, we can clearly see that mass spectrometric separation for the purposes of quantifying and verifying the result is eminently possible. Without requiring further process steps, this method permits the determination of starting concentration, elimination and reaction kinetics for oxidative wastewater treatment. With an acetonitrile portion of 25% and a slower acetonitrile-water gradient, baseline separation is also possible with a total analysis time of about 10minutes. The weaker elution from the SPE cartridge results in peak broadening, however, which has the result of reducing process sensitivity (data not shown).
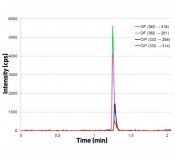
Fig. 2 Online SPE-LC-MS/MS method for the determination of ciprofloxacin (CIP) and ofloxacin (OF) using Spark Holland Symbiosis Pico and AB Sciex Q Trap 3200. (c = 1 ng/l, enrichment volume: 10ml, SPE cartridge: Spark HySphere C18HD, HPLC column: Phenomenex 50 x 2.1mm Kinetex 2.6µm C18 100Å).
Investigation and evaluation of transformation products
Both in ultrapure water and in treatment plant effluent, ozonation with 10mgO3/l achieved substance degradation of at least 80% for ciprofloxacin and 88% for ofloxacin. By using UV oxidation in combination with H2O2, complete substance degradation was achieved in treatment plant effluent. Follow-up investigations utilising QTRAP and TOF-MS were able to detect a total of 22 transformation products from ciprofloxacin and 15 transformation products from ofloxacin [5]. Effect-directed analysis was extended to antibacterial activity with a disk-diffusion test utilising the bacterial strains Pseudomonas fluorescens and Bacillus coagulans. Figure 3 shows the degradation of ofloxacin plus the simultaneous decline in antibacterial activity. The formation of inhibition zones around the applied sample can be clearly seen in both pre-treatment samples (photo below left and right). No inhibitory effects versus the two bacterial strains were observed in the blank array, the control samples and the samples after 30 minutes of oxidative treatment.

Fig. 3 Degradation of ofloxacin and reduction of microbiological activity by UV/H2O2 oxidation in spiked WWTP effluent. The photo shows the disk-diffusion test with B. coagulans (c0 = 50 µmol/l, B = blank array, -10 = sample before switching on UV lamp, 0 = sample after UV lamp warm-up phase, before addition of H2O2).
Summary
The results from instrumental and effect-directed analysis prove that substance degradation goes hand in hand with the loss of antibacterial activity. Considered alongside the results from cytotoxicity, genotoxicity and mutagenicity testing, an indirect conclusion may be drawn that the transformation products formed have no toxicological relevance in contaminated and uncontaminated real-world wastewater.
By combining instrumental and effect-directed analysis, it has been possible to confirm the working hypothesis that the types of procedures investigated (O3, UV and UV/H2O2) can be safely applied for the degradation of trace substances in real-world treatment plant effluents [5]. Since wastewater composition is not identical across multiple wastewater facilities, installation of large-scale ozonation plant should be preceded by guideline investigation work on ozonation and the formation of potentially relevant transformation products, conducted using instrumental and effect-directed analysis.
Literature
[1] �Proposal for a Directive of the European Parliament and of the Council amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy, COM(2011) 876 from 31/01/2012.
[2] �Türk, J., et al., Final Report on IGF Research Project No. 16145 BG, “Energy-efficient elimination of persistent pharmaceuticals from wastewater by using a pollutant-specific, pollutant-driven oxidation procedure (EPASGO)”, http://www.veu.de/files/abschlussbericht_igf_epasgo_16145_bg.pdfhttp://www.veu.de/files/abschlussbericht_igf_epasgo_, 2012.
[3] �Hartmann, A. et al. (1999) Arch. Environ. Contam. Toxicol. 36, 115-119.
[4] �Sanchez, G. et al. (2005) Photochem. Photobiol, 81, 819-822.
[5] �Türk, J., et al., Final Report on IGF Research Project No. 15862, “Investigating methods for the evaluation and avoidance of toxic oxidation by-products generated by oxidative wastewater treatment”, http://www.veu.de/files/abschlussbericht_15862.pdf, 2011.
|




















