|
Bio&Biotech
 >
Viruses in the water
>
Viruses in the water
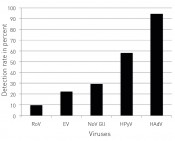
Viruses in the waterDetection and analysis of human viral pathogens in surface watersAlongside the development of methods for quantifying human viral pathogens in surface and waste waters, a key role is also played by the analysis of the resultant data. While drinking water supplies must be safeguarded on the one hand, the public must also be able to pursue waterrelated recreation activities without fearing health risks. Reducing viruses in waters of the Ruhr Within the Ruhr region, drinking water for an estimated 5.13 million people is supplied by the Ruhr, a tributary of the Rhine. It is also used by many of the region’s residents for leisure and recreation activities. Located in western Germany, the Ruhr region is the fifth-largest urban agglo meration in Europe. Home to major cities such as Dortmund, Essen, Duisburg and Bochum, it has a population density of over 1,100 people per square kilometre. The region’s high population density gives rise to significant contamination with anthropogenic substances and pathogenic organisms, the latter category including both bacteria and parasites as well as human pathogenic enteric viruses. For surface waters, potential contamination routes include diffuse sources and urban drainage systems. While current research is investigating options for reducing viral and bacterial load in treatment plants, there is a lack of usable data about the role played by treatment plants and diffuse sources – such as arable field and grassland run-off – in the contamination of surface waters with microorganisms. That said, the detection of enteric viruses at sites several kilo metres downstream of the treatment plant has been confir med under conditions of 15 °C and less. As over 60 treatment plants discharge waste water that is treated but still contaminated with viruses along the length of the river, these highly persistent viral loads can accumulate over the course of the river at low temperatures. The hy gienisation of treat ment plant effluent is therefore of parti cular importance. One method investigated is ozonation, which has mainly been used by drinking water facilities. Yet adapting this technique to sludge treatment is not entirely straightforward, since the effectiveness of reducing the microbiological load is crucially dependent on ozone concentration and dosage, temperature and pH values. One must also bear in mind that the ozone concentration was controlled via SAC 254 in the treatment plants investigated, i.e. both the dose and input mode were selected to ensure no residual ozone was detectable in effluent. Under these conditions, viral detection via PCR is especially proble matic, since the ozone first damages the viral capsid before damaging the genome. Accordingly, false positive results may be generated, since the methods for quantifying viral DNA do not take into account the possibility of viral capsids suffering damage – which corresponds to a loss of infectivity [1]. Method of investigation In recent years, increased interest has been shown in the enteric viruses – e.g. noroviruses, rotaviruses and enteroviruses – from both a scientific and public health perspective. While drinking water facilities can efficiently reduce those viruses, water sports enthusiasts in particular still face a previously unconsidered risk of infection. Unlike bacteria, the abovementioned nonenveloped enteric viruses are characterised by being both highly infectious and highly stable in the environment. As few as 10 – 100 viral particles are sufficient to cause infection. In a project funded by the German Federal Ministry of Education and Research (BMBF), 180 samples from eight different locations around Lake Baldeney in Essen were tested and the concentrations of five human enteric viral pathogens were determined using quantitative real-time PCR – the current method of choice for detecting and quantifying enteric viruses in water – and methods from molecular bio logy. Since viral concen trations in surface waters are too low to be detected directly, the environmental samples – typically 10 to 60 litres – first needed to be concentrated using the „Virus Absor ption and Elution“ (VIRADEL) method [2]. This specialised filtration method utilises a filter membrane that is charged either electropositive or electrone gative. Since the viral capsid and the filter membrane carry opposing charges, the viruses are captured effectively and thus retained. The advantage of this filtration method derives from the fact that the viruses can be eluted by utilising a specialised buffer solution, whereby the viruses are concentrated in 100 ml. In the next step, the eluate is again reduced to a maximum of 5 ml, ready for subsequent quantification following DNA/RNA extraction by utilising techniques from molecular biology. To be able to make statements about the danger to health present for sports enthusiasts and bathers using the Ruhr, however, it is necessary to determine the concentration of infectious viruses using cell cultures. In this process – which is time-consuming and not available for all viruses – monolayer cultures of animal cells are applied to nutrient media under sterile conditions and then inoculated with environmental samples. Viruses that are infectious proceed to infect the cells: the magnitude of the infection can be used to quantify the concentration of infectious viral pathogenic organisms contained in the water sample [3]. Risk assessment for viral incidence Key findings from the testing of Ruhr water for enteric viruses are that the noroviruses (family: Caliciviridae, genus: Norovirus), en teroviruses (family: Picornaviridae) and rotaviruses (family: Reoviridae, genus Rotavirus) are of especial significance from the perspective of pathogenicity, although detected in samples comparably less often than the adenoviruses and polyomaviruses (fig. 1).
Fig.1: Detection rates of human viral pathogens in percent: adenoviruses and polyomaviruses are detected in Ruhr water samples in over 90% and over 50% of cases, respectively. Noro-, rota- and enteroviruses are more significant in terms of pathogenicity. Legend: HAdV – human adenoviruses; HPyV – human polyomaviruses; NoV GII – GII noroviruses; EV – enteroviruses.
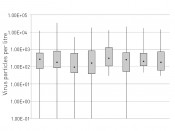
Unlike the other viruses measured, adenoviruses and polyomaviruses cause latent infections, and are excreted continuously from infected individuals. As a result, these viruses are detectable in surface waters without significant seasonal fluctuations. For rota-/noroviruses (principally found in water during the cold months of the year) and enteroviruses (primarily found in water during the warm months), the situation is different (fig.2).
Fig.2:Concentration of adenoviruses at the 8 sampling sites: no significant variance is observed in adenovirus concentration among the individual sampling sites. Legend: Points – median; box: 25 th to 75 th percentiles; whiskers – minimum and maximum values
Tab.:Comparison of average quantity of water ingested while bathing. As they are more physically active, boys swallow more water than girls and children/adolescents swallow more water than adults (6).
In considering the ramifications for health of the viral load present in surface waters, a quantitative microbial risk assessment (QMRA) is a useful analytical tool. QMRA calculations are based on parameter-specific dose-response curves that can be mathematically described using equations 2 and 3. For the project described, the dose is calculated from the time spent swimming, the quantity of water ingested and the pathogen concentration in water (equation 1).
D = C x T x R (Equation 1)
(Equation 3) Note that this risk assessment does not supplant the efforts of the World Health Organisation as regards its Water Safety Plan [4], which requires a preventive approach to risk minimisation to safeguard a high level of drinking water quality. Instead, this form of risk assessment could be considered in the future for use in a risk management model that can be applied when evaluating new conditions (e.g. construction of new waste water facilities, changes to land use, etc.). This could have implications both for drinking water production and for the use of a watercourse as a local recreational area. Moreover, the QMRA could also be deployed to establish microbial threshold limits for microbiological parameters not yet taken into account by legislative measures. Complex diagnostic procedures mean viruses have little value as routine parameters. In the EU Bathing Water Directive (2006/7/EC), quantification of the faecal indicators E. coli and enterococci are stipulated as a quality criterion. Since bacteria are less resistant and persistent, they are unsuited for use as indicators of viral load. For detection of the phage oX174 with E. coli as host, a DIN-certified detection method would be available – a method that would even permit statements to be made about its infectivity. Since no correlation exists between the occurrence of these phages and other enteric viruses, however, the former does not constitute a reliable indicator for human viral pathogens [5].
Bibliography [2] Hamza, IA. et al. (2009) Detection of human viruses in rivers of a densely-populated area in Germany using a virus adsorption elution method optimized for PCR analyses. Water Res 43:2657–68 [3] Hamza, IA. et al. (2011) Methods to detect infectious human enteric viruses in environmental water samples. Int J Hyg Envir Heal 214:424–36 [4] WHO. 2005. Water Safety Plans – Managing drinkingwater quality from catchment to consumer. pp. 1–225. Geneva: Davison, A.; Howard, G.; Stevens, M.; Callan, P.; Fewtrell, L.; Deere, D.; Bartram, J. [5] Jurzik, L. et al. (2010) Chemical and microbiological parameters as possible indicators for human enteric viruses in surface water. Int J Hyg Environ Health 213:210–6 [6] Dufour, AP. et al. (2006) Water ingestion during swimming activities in a pool: a pilot study. Journal of Water and Health 4:425–30 Picture: © panthermedia.net | knape |
L&M int. 3 / 2014
Free download here: download here The Authors:Read more articles online |