|
Scientist
>
Prof. Dr Michael Rychlik
>
How can we safeguard the quality of our beer?
How can we safeguard the quality of our beer?From the barley to the bottleBeer is an integral part of German culture. Not only because of the German "Beer Purity Law" (Reinheitsgebot) it is that we associate the drink with concepts such as “traditional”, “wholesome” and “additive-free”. As a cereal product, however, beer is subject to the same conditions that affect the cultivation, storage and processing of other cereal crops. One of the problems with which almost all cereal products are confronted is fungal contamination and the associated potential impairments to quality that this implies. The most important fungal genus here is Fusarium, which preferentially infects cereals during the flowering period. Other causes are the dematiaceous (pigmented) fungi, which spoil cereal grains affected with moisture during ripening or storage. While wheat is particularly prone to infestation, brewer’s barley can also be affected (fig.1). In the 2007 harvest, for example, an extremely high level of Fusaria contamination was observed, accompanied by an unusually set of symptoms (esp. discoloured red and black grains, see fig.2). In 2010, major contamination with dematiaceous fungi was observed in barley at harvest, while the occurrence of discoloured red grains was so frequent in some wheat harvests that large parts of this crop proved unsuitable for use in brewing.
Fig. 1: Ears of barley blighted by Fusarium avenaceum (left) compared to healthy control (right)
Fig. 2: Discoloured red barley grains blighted by Fusarium (top) compared to healthy controls (bottom)
Risks posed by Fusaria and their metabolites In the case of barley, no effective fungicide or resistant strain is available for managing Fusaria-induced cereal blight. Yet infection with Fusaria can dramatically impair the quality of unmalted barley grains. One problem brewers ascribe to the metabolites produced by Fusaria (known as hydrophobins) is “gushing”, a highly unwelcome phenomenon in which the beer spontaneously gushes out of the bottle as soon as it is opened without shaking the bottle. Fusaria are also responsible for producing an array of mycotoxins, although only deoxynivalenol (DON) has been given a maximum limit in the EU to date. In cereal crops intended for further processing before consumption, a value of 1250µg DON/kg is tolerated. For beer, where we assume the addition of 10% malt on average, the corresponding maximum limit is 125µg DON/l. Along with the metabolites produced directly by fungi, foodstuffs can also contain “modified mycotoxins”, one example being DON-3-glucoside. This compound is formed by plant metabolism from the original toxins [1]. These “modified mycotoxins” can either be toxic themselves or their degradation in the human gastro-intestinal tract may release the original toxin. As such, these substances may pose additional risks, even though they are not legally restricted or even fully accessible to lab analysis. In addition to the abovementioned DON and its glucoside, Fusaria may also form other toxin groups, including other trichothecenes, zearalenone, moniliformin, enniatins, beauvericin and fumonisins. The detection and analysis of Fusaria contamination is extremely difficult, both in the field and in the supply chain to the brewery. To date, the contamination or level of contamination with moulds has primarily been assessed using manual scoring (also used to detect grain anomalies such as germination or second growth), i.e. using only visual inspection as the basis for drawing conclusions about the degree of contamination present. This method takes only the visible set of symptoms into account, however (visible mould growth – causing red, white, salmon-pink or black grains): contamination that does not cause discolouration remains undetected. Solution: a systematic analysis of Fusaria contamination The Chairs of Brewing and Beverage Technology, Phytopathology and Analytical Food Chemistry at Technische Universität München have therefore joined forces with the aim of systematically establishing the links between the type and degree of Fusaria contamination (spectrum of Fusarium species), symptoms (e.g. red and black grains) and metabolites (mycotoxins, key proteins, chemical defences) across the entire process, from raw grain to finished product. Experiments consider both typical samples of real-world barley, so as to establish the status quo, as well as specifically inoculated sample material (greenhouse inoculation = single inoculation (contamination) with one Fusarium species) and field-plot inoculations (mixed inoculation with one primary species), so as to generate a comprehensive and systematic set of data. Selection of the Fusarium species is based on differentiation of their toxin-forming potential. Impairments to qualities important for processing (solubility in the malting process with a focus on the proteolytic solution) as caused by fungal contamination will also be investigated. The Fusaria contamination or microbial blight present will be precisely differentiated by both conventional and molecular methods of phytopathological analysis. Alongside molecular diagnostics, the degree of contamination and the symptoms that result will be recorded using the manual scoring typical in the industry. The Fusaria and their related metabolites toxins formed and their metabolites will be quantified using LC-MS/MS and a combined method using stable isotope dilution assays (SIDAs) and matrix-matched calibration. Supplementary gene expression studies from re-inoculated samples will help researchers to obtain indications of the production of metabolites relevant for the steps in the malting process. The investigation of the links between Fusaria contamination, symptoms and mycotoxin pollution of the brewer’s barley starting material from the malting process to the final malt product – and ultimately the beer itself – is intended to improve both quality controls and product safety.
Fig. 3: Greenhouse-cultivated barley following inoculation with Fusarium
Fig. 4: Germinating barley during the malting process
Fig. 5: Capillary electrophoresis (lab-on-chip with Offgel®, two-dimensional gel electrophoresis), which enables a protein characterisation of the malts
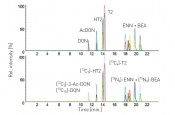
Fig. 6: LC-MS/MS chromatogram as part of the multi-stable isotope dilution analysis (SIDA) for quantifying the most important Fusarium toxins
Fig. 7: LC-MS/MS chromatogram of a malt extract with detection of deoxynivalenol (DON) and the modified DON-3-glucoside mycotoxin
Findings to date For the systematic production of the specific barley, malt and beer samples, the first step was for the Chair of Phytopathology to develop a procedure for the reproducible inoculation of barley plants in the greenhouse (fig. 3) and on field plots. The for Brewing and Beverage Technology then malted (fig. 4) and brewed the individual inoculated samples in order to provide a kind of in-process check on the technological steps. A pilot study was used to successfully test this procedure with the sample substances of beauvericin and the enniatins [2]. Capillary electrophoresis was also set up for protein separation and documentation, thus serving to assess the quality characteristics of the malt (lab-on-chip with Offgel®, see fig.5). Finally, the of Analytical Food Chemistry then integrated the most important Fusaria toxins in barley into a multi-SIDA in which SIDAs were combined with matrix-matched calibration (fig.6). Findings from the project to date based on specimens from a brewers monitoring for quality of the raw material and inoculated samples discovered poor correlation between manual scoring (i.e. red and black grain counts) with the DNA of Fusaria considered by the project to date and the toxins these species produce, namely DON and T-2/HT-2 toxin. In the specifically inoculated barley samples, the DNA of the corresponding species was quantified, as were known toxins: Fusarium culmorum and F. graminearum produced DON, F. sporotrichioides and F. langsethiae produced T-2 and HT-2 toxin, and F. avenaceum and F. tricinctum produced enniatins and beauvericin. For producers of the type B trichothecene group, it was also discovered that the modified mycotoxin DON-3-glucoside was generated from DON especially during malting, in quantities far exceeding the quantity of DON itself (fig.7). From the malts generated in the project, beer is being brewed and toxins are being tracked through the individual sub-processes. In the future, this will permit the investigation of strategies to guarantee high-quality beer.
Bibliography Photo: © istockphoto.com| Man_Half-tube , flavijusflavijus |
L&M int. 2 / 2015
Free download here: download here The Authors:Read more articles online |