|
L&Mint-int-3-2015
>
Cell experiments with optical tweezers are revolutionising biomedicine
Cell experiments with optical tweezers are revolutionising biomedicineTweezing without touchingUltramodern imaging techniques such as the Nobel Prize-winning STED microscopy enable the investigation of organisms, cells, bacteria and even viruses, DNA or individual molecules at very high spatial and temporal resolution. Active intervention in these tiniest of biological structures has been largely limited to indirect methods, however. While new developments such as microtweezers and micromechanical clamps are promising, these devices generally tend to alter the properties of the objects to be investigated, making meaningful measurements difficult – especially in vivo. Focused laser beams, on the other hand – known as “optical tweezers” – permit the contactless manipulation of cells and bacteria in three three dimension of space. Viscoelasticity: an essential feature of both health and disease Many of life’s processes depend on the spatial and temporal interaction of proteins or cells as fundamental building blocks. Studying these interactions is an essential part of almost all areas of disease research – especially for work on infectious diseases and cancer – and is closely related to the question of the cell’s biomechanical properties. Many cellular processes depend on the viscoelasticity of cells and their environment. An object is said to be “viscoelastic” if it exhibits both the elasticity found in solid objects (such as rubber) as well as the viscosity found in liquids (such as honey). During different cellular processes, as .e.g cell division, infection or cell death, the viscoelasticity of cells can undergo significant modifications. In coronary heart disease, for example – easily the most prevalent cause of death worldwide – the build-up of deposits on the inner wall of blood vessels causes these vessels to lose their elasticity [1,2]. The relationship between cellular processes and biomechanical properties therefore holds the keys to many questions in biomedical research. Knowledge of the changes to viscoelasticity occurring between and within cellular processes can improve our understanding of important modern diseases – including Alzheimer’s and tumour growth – and help us to develop medications to treat them. Any investigation of cell viscoelasticity requires us to deform these cells and take measurements that quantify the force required for this deformation. Accordingly, we need to develop a technique with which we can repeatedly deform a cell with a predefined force. In 1970, Arthur Ashkin showed that light can be used to exert a force on objects [3].
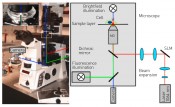
Fig. 1: Optical tweezers, integrated into a microscope (left). The detail shows the sample layer. In the microscope, the optical tweezers can be combined with various modern high-resolution microscopic techniques, such as dark-/bright-field microscopy, fluorescence or confocal microscopy and quantitative phase-contrast microscopy. Two lenses are used to widen the laser beam, which then illuminates the light modulator. Two further lenses are then used to guide and focus the beam onto the microscope objective.
Holographic optical tweezers As early as the 1600s, Johannes Kepler had already hypothesised that the tail of a comet was caused by light pressure accelerating the comet’s dust particles to form the tail. The tail points away from the sun, i.e. in the same direction as the propagation of light. The force moving objects in the light’s direction of propagation is termed “scattering force” and is proportional to light intensity. For transparent objects and inhomogeneous intensity distributions, an effect termed the “gradient force” also applies. This force is directed towards the point of highest light intensity. A large gradient must be present in order to move objects towards this point. This can be achieved by focusing the light beam, typically using strongly focusing microscope objectives with a high numerical aperture. The numerical aperture defines one half of the aperture angle of the light cone exiting the objective. This angle – and thus the gradient force – increases proportionally with the numerical aperture. If light intensity is sufficiently high, the gradient force exceeds the scattering force, pulling transparent objects towards the focal point and trapping them stably there in three three dimension of space. In 1986 Arthur Ashkin worked with subsequent Nobel Prize winner Steven Chu to develop the first gradient light trap based on this principle [4], later to become more widely known as “optical tweezers”. This approach not only lets us trap transparent objects with the aid of a focused laser beam but also allows us to move them in three three dimension of space. Objects with a size of several microns can be trapped in this way, that process is ideal for cells and bacteria. Optical tweezers are typically integrated into existing microscopy techniques thus enabling the objects to be manipulated and observed simultaneously. Since the manipulation of the trapped objects is also both contactless and sterile, this offers biological and medical applications a key advantage when compared to conventional methods for micromanipulation. To characterise the manipulation appropriately, it is necessary to determine the forces actually created by the optical tweezers. To do so, the position of the trapped object is compared with the focal position of the optical tweezers. If no additional force is acting on the object, the object is at the focal point. If a bacterium (for example) attempts to move with the aid of its flagellum, then the bacterium itself exerts a force countering the optical force exerted by the optical tweezers. This enables the bacterium to remove itself slightly from the focal point while still being confined in the optical trap. The distance from the focus to the bacterium is proportional to the force generated by the bacterium and can thus be determined by positional measurements. Optical tweezers are especially useful for measuring minute forces at the piconewton (pN) scale within cells and between single biological cells. These forces are at the same scale as those acting on and within a cell. As an example, intracellular nutrient transport is achieved by molecular motors with a force of about 5pN. Optical tweezers make it possible to trap just one object at a time. To characterise viscoelasticity, it is necessary to immobilise and then deform a cell. Multiple traps are therefore required and it must be possible to move them dynamically. We achieve this by making use of holographic methods. Holograms contain three-dimensional information describing the position of the traps in the focal plane. We use spatial light modulators (SLMs) to impose the hologram onto the laser. A light modulator is a liquid-crystal display that performs per-pixel modulation of the laser beam to enable the presentation of an arbitrary image. This approach transforms the optical tweezers into holographic optical tweezers (HOT). HOT can manipulate a large number of traps by modulating a single laser beam. Figure 1 presents a schematic overview of a HOT system integrated into a microscope. Examples of typical holograms for generating a variety of trap configurations can be seen in Figure 2. This offers us a technique satisfying all of the requirements for the characterisation of viscoelasticity in the context of disease research.
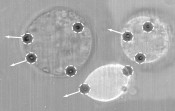
Fig. 2: The specific hologram used (shown in the upper row) determines both the layout and number of traps (shown in the bottom row). A grating-like pattern displaces the traps laterally, while a lens-type pattern displaces the traps axially. By combining multiple gratings and lenses, a single laser beam can be used to generate multiple traps, which we can arrange three-dimensionally and vary dynamically over time. These traps can be manipulated independently of one another and simultaneously.
Intracellular viscoelasticity and mobility To investigate the viscoelasticity of cells, tissue and blood vessels, we need to understand the involvement of individual cell components and organelles in this process. Considering the cell as a whole, viscoelasticity is a product of several effects stemming primarily from the cell membrane, cytoplasma and cytoskeleton, but also involving other cellular components. Since most of the cell’s biomechanical properties are determined by its cytoskeleton, this structure is of particular interest [5]. To characterise the viscoelasticity of the cytoskeleton, the dense network of filaments in the cell needs to be stretched and deformed. Deformation also results from the natural movements of cell organelles such as vesicles, for example. We also introduce particles into the cell plasma of living cells. By using HOT to move the particles within the cell, we can draw conclusions about the cytoskeleton and thus characterise the interior of the cell itself [5]. The same experiment can also be used to determine the viscoelasticity of the cell membrane. To do so, we need to guide the particles within the cell plasma towards the cell membrane. Figure 3 shows three cells in which particles have been pressed against the cell membrane from the inside, resulting in obvious stretching. In these cases, HOT are used as a holographic optical “stretcher”. With the help of HOT, we can deform a large number of cells. This technique also stretches the cytoskeleton, enabling us to examine its influence. This makes it possible to examine cells specifically and locally at multiple locations, and to identify differences in viscoelasticity within a cell or a group of cells. The interplay between cellular processes and cell viscoelasticity can also be investigated. This enables us to take a systematic approach to study the biomechanical mechanisms of disease.
Fig. 3: Deformation of multiple living cells. Multiple traps (white circles) are deployed with the aim of deforming the membranes at multiple locations in different directions (shown by arrows), so as to determine local viscoelasticity. Cell elasticity is proportional to the degree of deformation. Many diseases cause cells to lose their elasticity. Here, we typically see major differences in cell deformability.
Conclusions: interventional options for biomedicine
The use of HOT lets us trap and manipulate cells or bacteria with light. Two fundamental forces – scattering force and gradient force – are
Bibliography Picture: © istockphoto.com| Rich Legg |
L&M int. 3 / 2015
Free download here: download here The Authors:Read more articles online |