|
Chromchat
 >
Pharmaceutical counterion determination
>
Pharmaceutical counterion determination
Pharmaceutical counterion determinationHouse lights down, stage lights upIn contemporary practice, roughly half of all active pharmaceutical ingredients (APIs) are administered as salts. The use of the protonated or deprotonated form of the drug substance combined with the selection of counterions enables the targeted variation of key parameters, such as solubility and stability. Analysis of the corresponding counterions constitutes an essential part of the development process for new pharmaceuticals and is now an indispensable procedure within the quality control process for these products.
Fig. 1 Schematic presentation of cation and anion functional groups in Thermo Scientific™ Acclaim™ Trinity P1 mixed-mode columns.
Increasingly sophisticated techniques – such as combinatorial synthesis – are now being applied in the search for new APIs. Apart from modern chromatographic approaches such as ion chromatography and HPLC, counterion analysis has been tended to be left in the shadows to date. Often, the methods and techniques deployed are not particularly powerful. By optimising separation conditions and detection technology, HPLC offers a modern, high-output alternative: the result is automated counterion analysis that is more efficient and more powerful – reflecting the state of current technology standards. Contemporary methods of counterion determination In the pharmaceutical lab, we principally find potentiometric titration or ion chromatography (IC) used for counterion determination. Titration is both time-consuming and labour-intensive. While ion-exchange chromatography can analyse multiple anions or cations within one measurement, IC is nonetheless unable to analyse anions and cations simultaneously on a single stationary phase. Examples of highly-specific methods for cation analysis include spectroscopic methods such as inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS). Yet both of these methods are limited to a narrow range of applications while also being expensive to implement. All in all, none of the methods mentioned offers the option of analysing a series of ions with different charges during a single analysis run. Ion-exchange chromatography is certainly a powerful form of liquid chromatography that offers detection functions specific to anion or cation analysis and enjoys widespread deployment. Yet modern pharmaceutical formulations are now exhibiting increasing complexity. This complexity increases further if we then include the field of biopharmaceutics. If both cations and anions are present in a single sample, then at least two separation setups will be necessary, and analysis on the basis of IC is correspondingly more complicated. Pharmaceutical counterion analysis tends to involve the analysis of more complex samples, requiring a methodology that is both flexible and straightforward. LC separation techniques for the simultaneous analysis of anions, cations and other compounds The potential of LC methods to separate out substances depends on the selective interaction of analyte molecules with the stationary phase, i.e. it results from their ”selectivity“. As a separation mechanism, ion-exchange chromatography is unrivalled in the separation of small ionic substances. It depends on the selective electrostatic interaction of charged analytes with functional groups bearing an opposing charge on the surface of the stationary phase. Alongside primary separation mechanisms, secondary interactions also play a decisive role in selectivity for all LC separation techniques. In the development of novel phases for LC, increasing attention is now being paid to the targeted application of multiple and distinct – yet virtually equivalent – mechanisms. These are ”mixed-mode“ phases. These mechanisms are termed ”mixed-mode“ phases. The first columns of this type combined reversed-phase (RP) mechanisms with cation or anion exchange. Although these phases provided novel kinds of selectivity while also permitting the simultaneous separation of ionic and neutral substances, they did not allow the simultaneous retention of hydrophilic cations and anions. The inclusion of functional groups for both cations and anions on a single phase is effective only if physical separation of both groups prevents their reciprocal deactivation as a result of internal salt formation. Thanks to an ingenious system of synthesis, this challenge was first overcome by the use of ”trimodal“ phases: the structure of such phases is illustrated by the diagram given in Figure 1. These innovative mixed-mode phases combine anion and cation exchange with the reversed-phase mechanism of separation or with hydrophilic interaction liquid chromatography (HILIC), for example. Nor do these trimodal phases merely permit the simultaneous analysis of positively- and negatively-charged ions: for most formulations in the traditional world of small-molecule pharmaceuticals, these systems also enable simultaneous determination of the active substance.
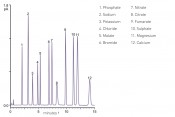
Fig. 2 Separation of anions and cations commonly used as pharmaceutical counterions using an Acclaim Trinity P2 column and detection with a Thermo Scientific™ Dionex™ Corona™ Veo™ RS charged aerosol detector.
Charged aerosol detection: it's universal Conventional detection procedures are unsuitable for exploiting the benefits offered by the trimodal stationary phases described above, however; since the vast majority of typical drug substance counterions lack chromophores, this rules out the use of UV detection. While conductivity detection is in fact suitable for such counterions, neither the variant without suppression of background conductivity nor the use of a suppressor system enables the direct simultaneous detection of anions and cations. At this point, one detection system can fully realise its potential, a system generally held to be a truly universal detection procedure within LC: charged aerosol detection (CAD). CAD begins with the nebulisation of the mobile phase, followed by the generation of a dry aerosol and the subsequent adsorption of ionised nitrogen onto the dried particle surface. In a final step, this charge is then measured within an electrometer. With full coverage of aerosol particles, this charge is proportional to the surface and is thus – as a first approximation – to its mass. This principle of measurement supplies truly universal detection proportional to the mass flow, capable of detecting both organic and inorganic anions/cations as well as neutral molecules. Therefore, in principle it is possible to detect all non-volatile or semi-volatile components of a pharmaceutical formulation.
Fig. 3 Chromatogram of the primary components of the drug Adderall® (Shire Pharmaceuticals), a formulation for the treatment of attention deficit disorder; saccharin and amphetamine are detectable using UV detection, aspartate, sodium and sulphate via charged aerosol detection.
An end-to-end counterion determination solution in modern pharmaceutical analysis and its applications It is self-evident that the combination of trimodal mixed-mode phases and charged aerosol detection provides the technical tools for a solution of this nature. The final touches to this technical solution are provided by the corresponding pre-programmed analysis setups (e-workflows) in the chromatography data system, which smooth the way to the result and analysis report with minimal training. The result is an all-in-one solution encompassing all of the key components and data, enabling even operators inexperienced in using LC to get off to a real ”flying start“. Figure 2 shows the deployment of this complete LC solution for the analysis of pharmaceutical counterions. During development of the stationary phase as utilised here, particular attention was paid to the simultaneous analysis of uni-/multivalent anions and cations. This apparatus makes it possible to separate and detect both inorganic and organic anions as well as univalent and bivalent cations within a single measurement. Overall, this makes it a simple matter to analyse twelve pharmaceutically relevant counterions within one gradient run in 15 minutes. At this juncture, it should once again be emphasised that the proposed method not only facilitates the simultaneous screening of multiple counterions but also makes it possible to analyse the API. Depending on the third retention mechanism preferred, operators have a choice of trimodal reversed-phase columns or HILIC retention. The proposed solution, consisting of a trimodal stationary phase and charged aerosol detection, is simple and straightforward to expand without forfeiting productivity. Figure 3 shows the separation of the primary components of a complete formulation. Note that this requires the analysis of volatile components, which exceeds the capabilities of the charged aerosol detector. Aspartate, sodium and sulphate are detected using the Corona Veo detector; for highly volatile amphetamine, a UV detector is used, connected in series. This approach ensures that an even greater spectrum of substances can be analysed with equal speed and simplicity.
For an in-depth illustration of the principle |
L&M int. 1 / 2014
Free download here: download here The Authors:Read more articles online |