|
science
 >
Near-infrared spectroscopy in pharmaceutical analysis
>
Near-infrared spectroscopy in pharmaceutical analysis
Near-infrared spectroscopy in pharmaceutical analysisGood vibrationsNear-infrared spectroscopy (NIRS) is based on the absorption of radiation by matter. Not only does the versatile technique allow the parallel identification of substances (active agents, excipients, contaminants), it is also suited to monitor processes such as blending, granulation, and drying.
Fig. 1 Applications of NIRS along the production chain.
NIRS – interaction of light and matter Molecular vibrations are induced in the near-infrared region of the magnetic spectrum (800–2500 nm) – i.e., from the end of the visible to the mid-infrared (MIR) range. The main absorption bands of the functional groups of chemical substances are located in the MIR range and are very strong. The absorption bands of the harmonics, and the combination of the fundamental molecular vibrations, however, are in the NIR spectral region. They are significantly weaker and enable direct measurement without sample preparation, while at the same time offering deep insights into the chemical and physical properties of the sample. The strongest overtone absorptions in the NIR range are displayed by compounds with OH, CH, NH, and SH bonds. Because the NIR spectrum represents the result of numerous overlapping absorption bands, it is normally evaluated with multivariate chemometric methods. Many parameters in a single analysis NIRS offers numerous advantages over many wet-chemical analytical methods. A diverse range of parameters can be determined simultaneously. NIRS is economical and fast enabling qualitative and quantitative analyses that are noninvasive and nondestructive. NIRS is an indispensable analysis technique that can be used along the entire production chain – from incoming materials to processing to the quality control of finished products (Fig.1). NIRS meets the requirements of numerous international pharmacopoeias, e.g., USP, Ph. Eur., and JP. Analyses of diverse matrices Near-infrared spectroscopy requires no sample preparation and can handle any sample matrix, whether it is powders or granulates, tablets or capsules, creams or gels, solutions or suspensions, polymer films, or freeze-dried samples. Screening through packaged materials NIRS can even perform determinations on contents sealed in transparent packaging such as glass and films. This is particularly appealing for incoming goods inspections and packaged end products. Handling is so easy that NIRS can be used directly in pharmacies and customs offices.
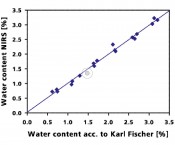
Fig. 2 Calibration model for quantitative determination of water content in powders. Karl Fischer titration is used as reference method.
Nondestructive analysis NIRS has long been one of the most important and versatile analytical techniques in the pharmaceutical industry – and not just because everybody in the pharma industry is talking about process analytical technologies (PAT) and Quality by Design (QbD). The decisive benefit of NIRS is the possibility of obtaining reliable analysis results in just seconds without altering the material under investigation and without any sample preparation or reagents whatsoever. PAT and QbD – in search of the best of all methods Drug manufacturing is subject to strong changes. The FDA's stated goal is to cut development time for new drugs while at the same time significantly improving quality. This requirement can only be fulfilled with analytical techniques that monitor the entire process – from incoming raw materials to the final product. To achieve that, perfect PAT sensors are needed that enable “live” tracking of the manufacturing process. NIRS is the technique that makes this possible. An inline sensor monitors product quality in real time. This prevents charges related to rejected products and reduces overall costs. An example: granulation and drying A key manufacturing process in the pharmaceutical industry is the granulation and drying process for powders that precedes tablet manufacturing. This process makes it possible to press powders into tablets in the first place. NIRS is the method of choice for determining the reaction endpoint when pressability is at the optimal point. Probes in the drier or granulator make it possible to track the process in real time. That reduces the process duration and thus increases the drying and granulation capacity of the system. At the same time, it minimizes the deviation of the required setpoint values. Figure 2 shows a calibration model for water determination that correlates NIRS to Karl Fischer titration which is the reference method. The progressive diminution of the water content during the granulation process, measured by real-time NIRS, can be seen in figure 3.
Fig. 3 Reduction of water content in a pharmaceutical powder over time. Rapid and nondestructive NIR analysis makes it possible to determine the optimal moment for further processing in real time. The sensor is installed directly in the granulator.
In accordance with international pharmacopoeias As a secondary test method, NIRS is recommended in all of the key pharmacopoeias – from the European (Ph. Eur. 2.2.40) to the American (USP<1119>) to the Japanese pharmacopoeia. Metrohm NIRSystems offers instruments that meet the standards for wavelength precision, reproducibility, and photometric noise. Numerous reference standards and the user-friendly software make it easy to verify the instrument’s compliance with requirements specified in the pharmacopoeias. The pharmaceutical version of the Vision software is fully validated and compliant with 21 CFR Part 11. Metrohm NIRSystems also offers complete IQ/OQ documentation and instrument performance certification (IPC). Documented parameters guarantee that the instrument performs properly. |
L&M int. 2 / 2014
Free download here: download here The Authors:Read more articles online |