|
Isothermal amplification in food analysis – a real alternative to conventional PCR
Isothermal amplification in food analysis – a real alternative to conventional PCRTrust, but verifyInherited genetic material – deoxyribonucleic acid or DNA for short – is unique to every living thing and can therefore be utilised like a fingerprint for identification purposes. DNA analysis has thus become an indispensable tool within food safety, and in the real-time testing of both raw materials and finished products. The established method for confirmation of specified target DNA in a sample is the polymerase chain reaction (PCR). In recent years, however, several more modern and simpler methods have started to appear, with serious potential to replace conventional PCR in certain fields while presenting at least an interesting alternative in many other areas of application. Isothermal amplification of DNA has ushered in a new generation of tests that are not only considerably faster than traditional PCR but potentially offer the option of being deployable outside a laboratory environment. If we glance at media reporting over the last few years we quickly come to the conclusion that many foodstuffs are not always what they appear to be. In addition, negligence or outright attempted fraud in the food industry can often result in serious repercussions for consumer health. While there are many methods available for detecting pathogens, allergens or other undesirable constituents, one problem is common to them all: they are expensive, time-consuming and must almost always be conducted under laboratory conditions. The rising demand for food products and an increased need for quality-tested foodstuffs continues to present both the food industry and regulators with new challenges. All of these stakeholders are clamouring for rapid, simple detection methods for specific DNA sequences. Question marks for dinner The label on a food product must specify its country of origin, expiry date and ingredients. We are given no information as to whether the product is contaminated with pathogens, whether all of its constituents are actually listed or whether the product contains substances that trigger allergies or have genetically-modified components. While many of the food products that end up on our table have already been analysed, tested and evaluated, this requires a laboratory and trained personnel capable of performing the relevant analytic procedures. There is a lack of rapid, simple detection methods capable of returning a result in less than a couple of hours. Listeria in dairy products In late 2009, the bacterium Listeria monocytogenes was discovered in sour milk cheese. Some 33 people contracted listeriosis, eight of whom died. This corresponds to a mortality rate of just under one in three patients, itself demonstrating the danger presented by contamination with these pathogens. The outbreak was caused by a failure to follow hygiene protocols and the use of out-of-date enzymes and other ingredients. Horsemeat in meatballs In 2013, Europe was shaken by the horsemeat scandal. Horsemeat was discovered in many meat-based ready meals – where it was naturally not listed as a product ingredient. One of the commonest drugs deployed within veterinary medicine – and thus by equine vets – is phenylbutazone, which is used for pain relief and to combat inflammation. The EU had already prohibited the use of this drug in animals destined for the meat production industry, since its use carries a number of health risks. The consumption of undeclared horsemeat can therefore have repercussions for health. Quite apart from the fact that it also constitutes outright fraud by mislabelling, since beef is considerably more expensive. Genetically-modified maize One topic that has attracted heated debate over the years is the addition of genetically-modified maize to a range of products and animal feeds. The risk posed to consumer health by the consumption of genetically-modified organisms (GMOs) has not been exhaustively investigated to date and therefore remains an open question. Notwithstanding this fact, many countries have introduced national legislation concerning the threshold value of GMO content above which a product must be appropriately labelled. Even in these cases, however, labels can still be economical with the truth. Allergens in food Although most allergens can be detected via antibodies using an ELISA (enzyme-linked immunosorbent assay) test, this method cannot be used in some situations. Celery offers one such example: it is too closely related to other plants such as the carrot and parsley, which are often included side by side with celery in recipes for sauces, condiments and pre-packaged meals. Celery has been classified as a potential allergen since 2005, however, and must therefore be declared on the label. This, in turn, can be achieved only with recourse to a DNA-based detection method. All of the potential ingredients named above are sourced from living organisms and therefore contribute their specific DNA to a product. Not only can this be used for specific detection but DNA also possesses key benefits compared to other analytes. It is highly stable, i.e. analysis is possible even for food that has been heavily processed. Even after prolonged boiling, DNA suffers only minor damage and can still enable positive identification. In addition, it can even distinguish between organisms that are very closely related – such as celery and parsley – and only a few copies are sufficient for reliable identification. Revolution in a test tube Kary Mullis developed PCR in 1983, thereby fundamentally changing the future of molecular biology and molecular diagnostics. From this point onwards, it became possible to make millions of copies of a single target DNA molecule within a few hours – thus facilitating its detection. One major disadvantage of conventional PCR is the necessity to use a thermal cycler, which must periodically heat, cool and re-heat the reaction mixture in cycles of a few seconds. The machine is not only expensive itself but incurs costs in terms of its maintenance and operation by trained personnel. In addition, the DNA, which is extracted from a complex sample matrix, must also be properly purified, since co-isolated inhibitors can disrupt the reaction procedure. Isothermal DNA amplification as an alternative to PCR The discovery of thermostable DNA polymerases exhibiting “strand displacement activity”, i.e. with the ability to take the DNA double helix apart by “unzipping it” and thus simultaneously permit the extension or de novo synthesis of an individual strand, has rapidly led to the development of many novel amplification techniques. One of the most fundamental benefits is the fact that these reactions can be conducted in isothermal – i.e. constant – conditions of temperature, therefore dispensing with the need for cycles at different temperatures. This property also obviates the need for the thermal cycler familiar from conventional PCR: instead, isothermal amplification reactions can be carried out on a simple heating block. Energy consumption for this constant-temperature process is also much lower than that required by alternate heating/cooling cycles, thus offering potential for a small, battery-operated instrument. LAMP: the isothermal racehorse One isothermal amplification method in particular has gained a stalwart following, namely “loop-mediated isothermal amplification” (LAMP) [1]. This method not only offers the appealing features of ruggedness and stability but is also very simple to perform (fig.1). The reaction itself is complex, however: six separate primers bind to the target DNA and modify the strand in such a way that the ends bind to themselves in loops, forming a dumbbell-like structure. As agglomeration of the primers to this structure successively continues, a longer and longer chain of consecutive target sequences is created, enabling the amplification of a tremendous amount of DNA in under half an hour. The advantage to this process is that the required reagents can be ready-mixed and freeze-dried beforehand: the only components then required to start the reaction are water and the extracted sample. The reaction vessel is simply heated to a predefined temperature and the reaction then starts immediately. After the prescribed period of roughly 30 to 45 minutes, a fluorescent intercalating dye is added. This type of dye can interact only with double-stranded DNA, which can itself be produced only by successful amplification of the target molecule. If this dye is able to insert itself between double-stranded DNA, then a colour change takes place from deep orange to fluorescent green (fig.2). Neither a UV lamp nor any other equipment is needed for detection: the colour change is clearly visible to the naked eye.
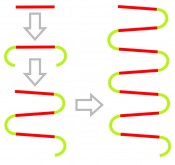
Fig. 1: Diagram of the isothermal LAMP reaction workflow. From the starting-point of a DNA target range (red), “loop” structures are then formed (green). Unlike PCR, LAMP does not result in the formation of a multitude of single, short products (amplicons) but in long product chains with continuously iterating sequences. The amplification of DNA is not cyclical but continuous: this results in the generation of a large quantity of product DNA. These fragments can attain lengths of over 20 kilobase pairs.
Fig. 2: Simple visualisation of test results from isothermal amplifications: the addition of a DNA intercalating dye causes positive samples to fluoresce.
“Pocket PCR” If there is no need to procure, maintain and operate a thermal cycler, this offers enormous savings in terms of time and money. One heating block measuring about 10x15cm is sufficient for performing an isothermal reaction and the result can be interpreted immediately using the naked eye (fig.3). The possibilities for developing tests for problematic issues such as horsemeat, bacterial contamination, GMO maize, etc. are almost unlimited. In the future, it should be possible to conduct such tests on-site: all that will be required for this is a small carrycase containing all of the necessary reagents and equipment, with which all of the procedures – from DNA extraction to the test results – can be performed in less than two hours.
Fig. 3: For isothermal amplification, only basic lab equipment is required, namely a pipette and a standard heating block. Sample colour change is readily identifiable: positive samples fluoresce bright green, negative samples retain their orange hue.
The Molecular Diagnostics Group at IFA-Tulln has already developed isothermal amplification tests for celery [2] and genetically-modified maize [3]. Current developments are focusing on distinguishing between different animal species (horse, cattle and pig) using these highly promising methods. All of these experiments indicate that this simple analytical procedure is the equal of conventional PCR both in terms of selectivity and sensitivity. The focus of future test development work will broaden from detection methods for foodstuffs to include tests for water. Simpler, faster tests would also be necessary in this area to guarantee the long-term safety of drinking water consumption – particularly in the developing economies. The possibilities here are almost unlimited and it is entirely conceivable that conventional PCR will be supplanted by novel, isothermal methods in many fields in a matter of years. Bibliography
[1] Notomi T. et al. (2000) Nucleic Acids Research 28(12), I–VII Picture: © Fotolia.com | photology1971 |
L&M int. 4 / 2014
Free download here: download here The Authors:Read more articles online |