|
Developing bio-based carrier systems for gene transfer
Developing bio-based carrier systems for gene transferGenes on sugarThe targeted transport of DNA and RNA using vectors (mostly made from synthetic polymers) in cell cultures has become part of routine practice in biological R&D – a fact highlighted by the multitude of commercial kits now available. To date, however, obstacles relating to use in patients have beset many laboratory studies and – in particular – the transfer to clinical practice. In many cases, such issues are related to safety concerns and limited biodegradability of such carriers. At FSU Jena, new bio-based and natural systems using polysaccharides are being developed with the aim of solving these specific problems. Vectors used to transfer DNA and RNA must be “all-rounders”, capable of performing a wide range of discrete tasks. These include maintaining the stability of their nucleic acid “cargo”, achieving an efficient and selective uptake in the target cells, and with high efficiency (transfection) while simultaneously ensuring excellent tolerability. Particularly in terms of use in patients, both their safety and their biocompatibility must be assured at all times [1]. Dextrans: familiar sugars, rediscovered Dextrans have been successfully deployed in pharmacy and medicine for many decades now as plasma expanders, tableting excipients, and stabilizers for colloidal formulations: both their toxicology and biodistribution are therefore adequately well-studied. The macromolecular biopolysaccharides are formed biosynthetically as a branched or unbranched molecule in sucrose-rich media by the activity of the dextransucrase enzyme, which is produced from various Leuconostoc strains. Dextrans are degraded in the organism and metabolized in the body [2]. Since they are not themselves capable of interaction with DNA and RNA, the logical next step was the covalent binding of cationic pendant groups to accomplish an electrostatic interaction with the negatively charged DNA and RNA [3]. To date, however, such side chains have often consisted once again of synthetic polymers rather than biogenic functionalities. Such polymers are neither biodegradable nor excretable and occasionally exhibit high toxicity.
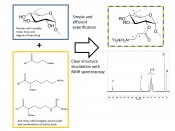
Fig. 1: Development of a dextran amino acid ester library with controllable, high-quality characteristics
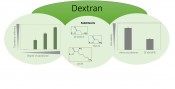
Fig. 2: Amino-acid substituted dextrans as biocompatible and highly efficient transfection systems
Sugars and amino acids: two natural partners In contrast to the above, introducing naturally-occurring functionalities with amine and ammonium groups appeared to be a highly promising approach. Work at Jena on polysaccharide chemistry has resulted in the development of efficient reactions under moderate conditions that permit the introduction of almost any carboxylic acid into polysaccharides [4, 5]. One pioneering achievement was the reproducible production of dextran amino acid esters with controllable molar mass and configurable content (average degree of substitution, DS) for the ester substituents – a synthesis variant that can easily be upscaled. In this approach, amino acids are activated with commercially available N,N-carbonyldiimidazole and undergo a homogeneous reaction to produce the corresponding dextran derivatives with a high level of purity and without appreciable polymer degradation (the average degree of polymerization (DP) of the initial dextran is preserved) or toxic byproducts. Based on this synthesis model, we gain access to a library of mono- and multifunctional dextran amino acid esters, which can also act as carriers of other functional groups: a construction kit for a practically unimaginable number of dextran derivatives offering considerable structural diversity. Products selected to date have exhibited outstanding characteristics as DNA transporters and interest is currently focused on ß-alanine-substituted dextrans. Alanine-substituted dextrans as DNA transporters Alanine-substituted dextrans are excellent water-soluble, can be stored permanently at room temperature following freeze-drying, and can be reconstituted quickly and completely in buffers and cell culture media. In standardized toxicity tests (according to DIN ISO 10993-5) using L929 mouse fibroblasts, they exhibited outstanding levels of tolerability that were higher by a factor of two than those for known synthetic polymers such as the commonly used polyethyleenimines [6]. If one investigates interactions with blood cells such as erythrocytes, which are the first contact partners within the organism following injection, no hemolytic effects or aggregations of blood cells – i.e. no thrombotic events – are observed in any therapeutically relevant quantities. The simple pipetting of DNA or RNA into the solutions of alanine-substituted dextrans causes the spontaneous formation of complexes that are deployable within a few minutes. With an overall cationic charge and a size of approx. 100nm under optimum conditions and batch ratios, the complexes meet all of the requirements for good uptake via the cell membrane. They can be deployed in serum-rich media and protect the nucleic acids against enzymatic attack from nucleases in blood just as well as they stabilize against the uncontrolled and undesirable displacement of the nucleic acids by serum proteins. They are not only capable of transfecting mammal cells but have the unusual property of causing no effects of toxicological concern even at high doses. What does the future hold? Modification of the complexes based on dextran-alanine for the intended application cannot be achieved merely by varying the production conditions and varying the formulation of the complex. The model can be re-used for a range of cationic amino acids and their combination. Properties such as the binding strength of the complexes with DNA and RNA, stability, toxicity and transfection efficiency can be controlled by the selection and combination of various types of amino acid. While lysine is more advantageous for binding DNA, for example, an excess of alanine has an especially positive effect on the transfection of cells. To sum up, the new synthesis strategy offers numerous approaches for structural variation. Amino acid-modified dextrans offer a simple way of bypassing numerous obstacles such as poor biodegradability, unacceptable toxicity and time consuming instructions for synthesis experiments.
Bibliography Picture: © istockphoto.com| George Clerk |
L&M int. 4 / 2015
Free download here: download here The Authors:Read more articles online |